Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help ! Question 3 only, the solution to Question 2 is = -352.293J Equation 3-5 is Mg(s)+2H^+(aq)+2Cl^-(aq) ---> Mg^2+(aq)+2Cl^-(aq)+H2(g) PART 1: DATA [2 POINTS]
Please help ! Question 3 only, the solution to Question 2 is = -352.293J
Equation 3-5 is Mg(s)+2H^+(aq)+2Cl^-(aq) ---> Mg^2+(aq)+2Cl^-(aq)+H2(g)
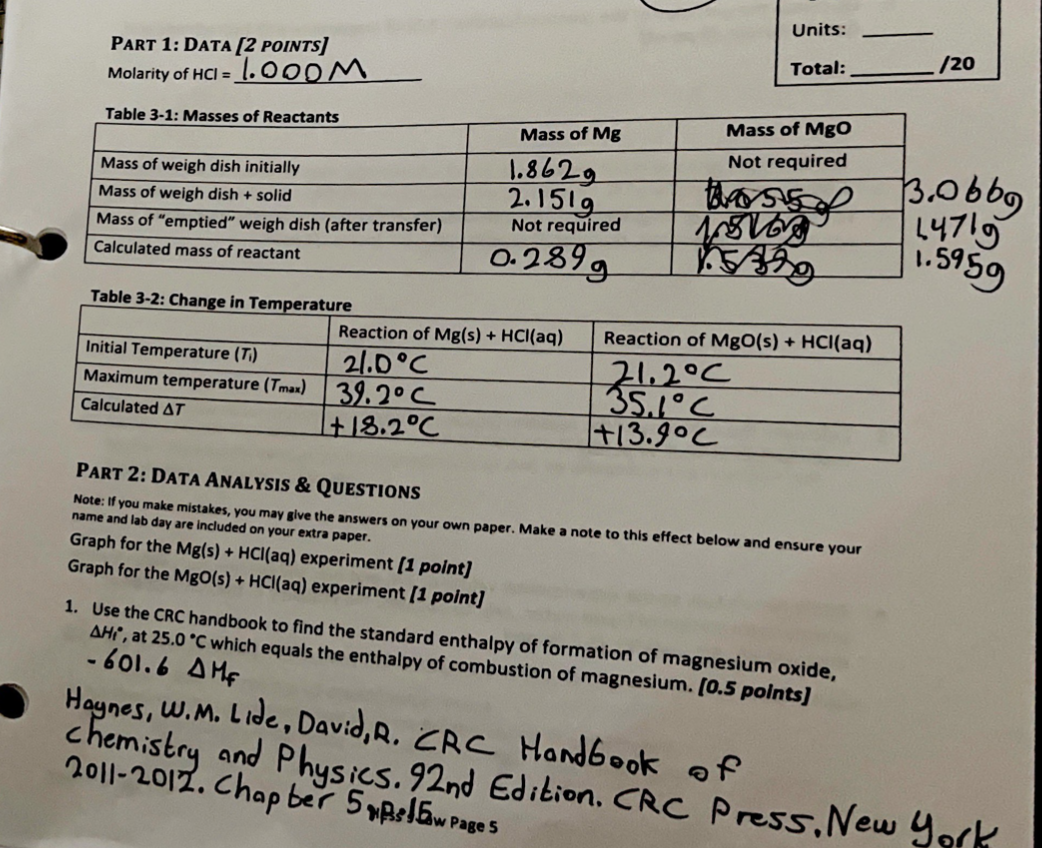

PART 1: DATA [2 POINTS] Units: Molarity of HCl=1.000M Total: 120 PART 2: DATA ANALYSIS \& QUeStIONS Note: It you make mistakes, you may give the answers on your own paper. Make a note to this effect below and ensure your Graph for the Mg(s)+HCl(aq) experiment [1 point] Graph for the MBO(s)+HCl(aq) experiment [ 1 point] 1. Use the CRC handbook to find the standard enthalpy of formation of magnesium oxide, Hi, at 25.0C which equals the enthalpy of combustion of magnesium. [0.5 points] 601.6MF Haynes, w.M. Lide, David,R. RC HandGook of Chemistry and Physics. 92nd Edition. CRC Press. Vew Yncl 2. For the reaction of magnesium with hydrochloric acid, calculate the heat of the solution (qsoln) and thus, the heat of reaction (q1n) in Joules. [1 point] 3. Calculate the enthalpy of the reaction (equation 3-5) of magnesium with hydrochloric acid, H1, in kJ/mol. [1 point]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


