Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help solve part B. Potassium perchlorate (KClO4) has a lattice Find the heat of solution for potassium perchlorate when 10.7g of potassium perchlorate is
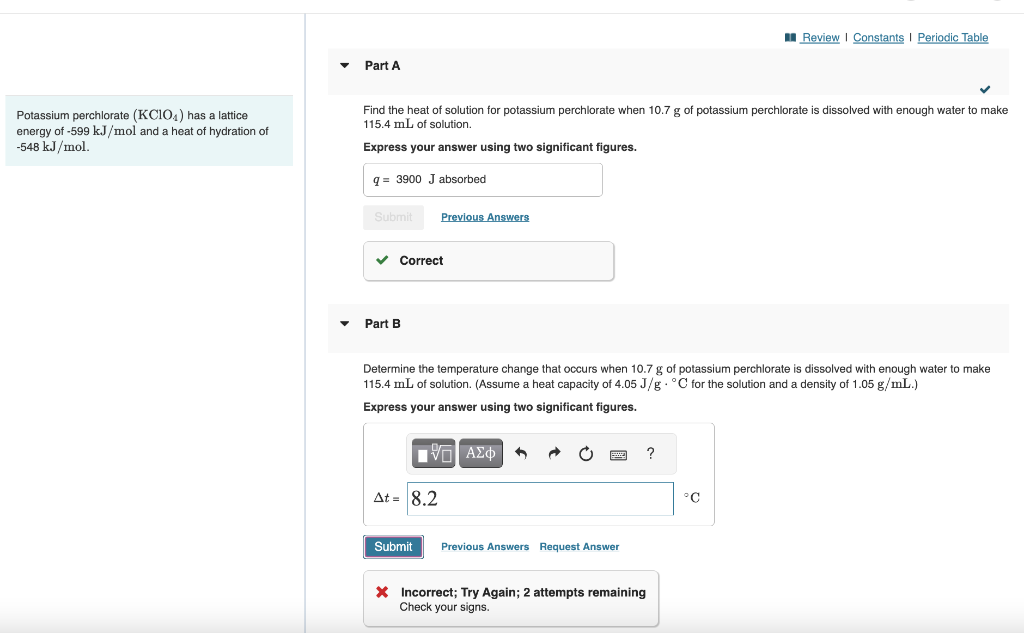
Please help solve part B.
Potassium perchlorate (KClO4) has a lattice Find the heat of solution for potassium perchlorate when 10.7g of potassium perchlorate is dissolved with enough water to make energy of 599kJ/mol and a heat of hydration of 115.4mL of solution. 548kJ/mol. Express your answer using two significant figures. Part B Determine the temperature change that occurs when 10.7g of potassium perchlorate is dissolved with enough water to make 115.4mL of solution. (Assume a heat capacity of 4.05J/gC for the solution and a density of 1.05g/mL.) Express your answer using two significant figures. X Incorrect; Try Again; 2 attempts remaining Check your signsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


