Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help. thank you 15. Find the enthalpy of reaction for this reaction CH3COOH+O2CO2+H2O using the bond energy values given below. Then draw the potential
please help. thank you 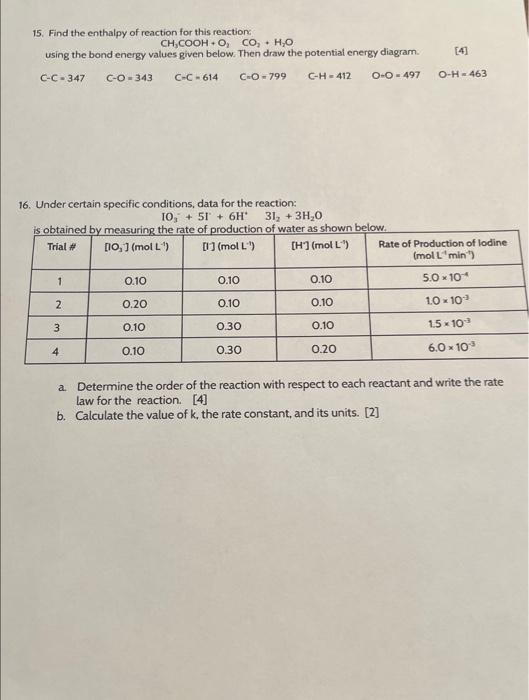
15. Find the enthalpy of reaction for this reaction CH3COOH+O2CO2+H2O using the bond energy values given below. Then draw the potential energy diagram. [4] CC=347CO=343CC=614CO=799CH=412OO=497OH=463 16. Under certain specific conditions, data for the reaction: IO3+5I+6H3I2+3H2O a. Determine the order of the reaction with respect to each reactant and write the rate law for the reaction. [4] b. Calculate the value of k, the rate constant, and its units. [2] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


