Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help! thank you What is the percent yield of a reaction in which 215.7g of phosphorous trichloride reacts with excess water to form 127.9g
please help! thank you 
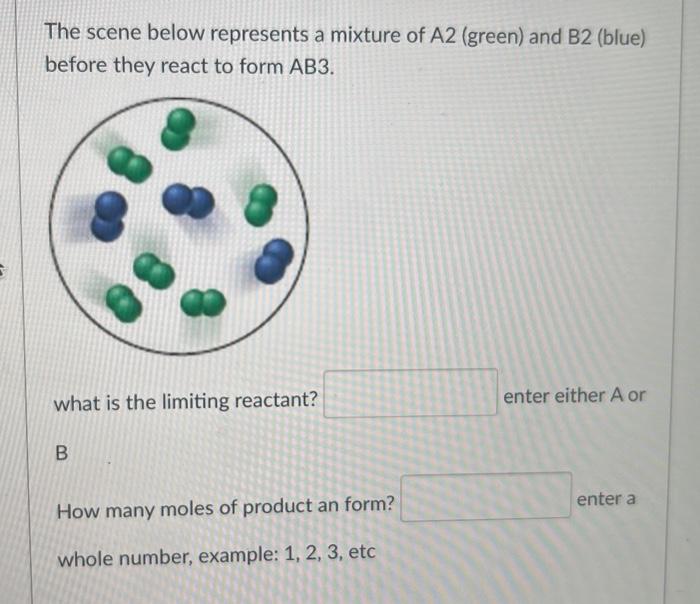
What is the percent yield of a reaction in which 215.7g of phosphorous trichloride reacts with excess water to form 127.9g of HCl and aqueous phosphorous acid (H3PO3) ? Enter to 2 decimal places. The scene below represents a mixture of A2 (green) and B2 (blue) before they react to form AB3. what is the limiting reactant? enter either A or B How many moles of product an form? enter a whole number, example: 1,2,3, etc 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


