Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please Help try to give accurate answers for the questions The following figure describes the particle size distribution in a dispersion of copper oxide particles
Please Help try to give accurate answers for the questions 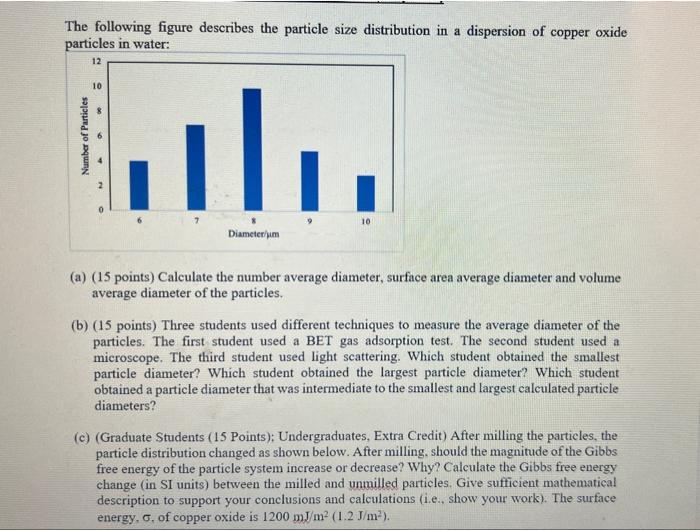
The following figure describes the particle size distribution in a dispersion of copper oxide particles in water: (a) (15 points) Calculate the number average diameter, surface area average diameter and volume average diameter of the particles. (b) (15 points) Three students used different techniques to measure the average diameter of the particles. The first student used a BET gas adsorption test. The second student used a microscope. The third student used light scattering. Which student obtained the smallest particle diameter? Which student obtained the largest particle diameter? Which student obtained a particle diameter that was intermediate to the smallest and largest calculated particle diameters? (c) (Graduate Students (15 Points); Undergraduates, Extra Credit) After milling the particles, the particle distribution changed as shown below. After milling, should the magnitude of the Gibbs free energy of the particle system increase or decrease? Why? Calculate the Gibbs free energy change (in SI units) between the milled and unmilled particles. Give sufficient mathematical description to support your conclusions and calculations (i.e., show your work). The surface energy, , of copper oxide is 1200mJ/m2(1.2J/m2). The following figure describes the particle size distribution in a dispersion of copper oxide particles in water: (a) (15 points) Calculate the number average diameter, surface area average diameter and volume average diameter of the particles. (b) (15 points) Three students used different techniques to measure the average diameter of the particles. The first student used a BET gas adsorption test. The second student used a microscope. The third student used light scattering. Which student obtained the smallest particle diameter? Which student obtained the largest particle diameter? Which student obtained a particle diameter that was intermediate to the smallest and largest calculated particle diameters? (c) (Graduate Students (15 Points); Undergraduates, Extra Credit) After milling the particles, the particle distribution changed as shown below. After milling, should the magnitude of the Gibbs free energy of the particle system increase or decrease? Why? Calculate the Gibbs free energy change (in SI units) between the milled and unmilled particles. Give sufficient mathematical description to support your conclusions and calculations (i.e., show your work). The surface energy, , of copper oxide is 1200mJ/m2(1.2J/m2) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


