Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help us 71 and 73 showing steps Determining Molecular Formulas Determine the molecular formula for each of the following. Show your work with units
Please help us 71 and 73 showing steps 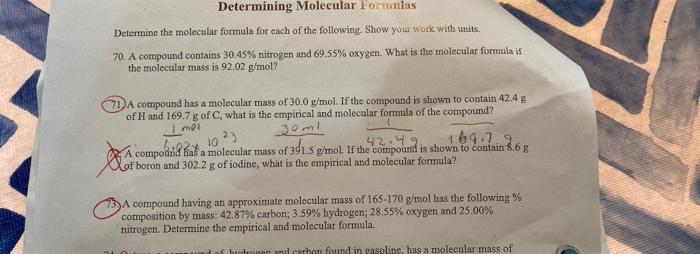
Determining Molecular Formulas Determine the molecular formula for each of the following. Show your work with units 70. A compound contains 30.45% nitrogen and 69.55% oxygen. What is the molecular formula if the molecular mass is 92.02 g/mol? A compound has a molecular mass of 30.0 g/mol. If the compound is shown to contain 42.4 g of H and 169.7 g of C, what is the empirical and molecular formula of the compound? 30ml A compodriah hata molecular mass of 301.5 g/mol. If the Compound 164.7.2 is shown to contain 8.6 g Imo 73 compound having an approximate molecular mass of 165-170 g/mol has the following% composition by mass: 42.87% carbon: 3.59% hydrogen; 28.55% oxygen and 25.00% nitrogen. Determine the empirical and molecular formula. of human and carbon found in gasoline has a molecular mass of 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


