Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with all :) At a certain temperature, 0.800 mol SO, is placed in a 4.00 L container. 2 50,(g) = 2 802(g) +
please help with all :) 
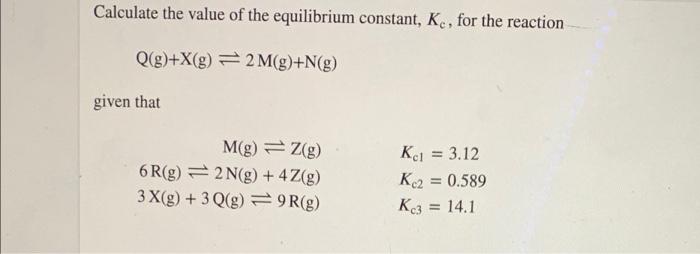

At a certain temperature, 0.800 mol SO, is placed in a 4.00 L container. 2 50,(g) = 2 802(g) + O2(g) At equilibrium, 0.140 mol O, is present. Calculate K. Calculate the value of the equilibrium constant, K, for the reaction Q(g)+X(g) = 2 M(g)+N(g) given that M(g) = 2(g) 6 R(g) = 2 N(g) + 4Z(g) 3X(g) + 3 Q(g) =9R(g) Kc1 = 3.12 K2 = 0.589 K3 = 14.1 Phosphorous pentachloride decomposes according to the reaction PCI,(9) = PCI,C) + C1, (8) A 13.7 g sample of PCI, is added to a sealed 1.50 L flask and the reaction is allowed to come to equilibrium at a constant temperature. At equilibrium, 47.0% of the PCI, remains. What is the equilibrium constant, K. for the reaction 
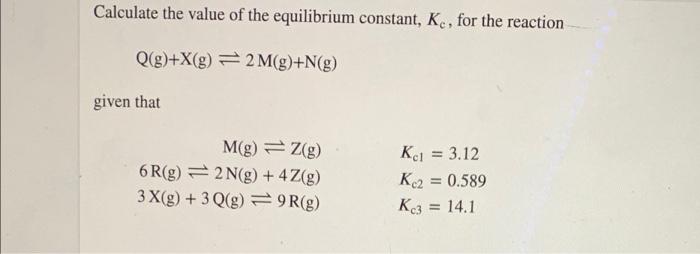

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


