Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with all parts 3. Consider each of the reactions of both acid compounds A & B with sodium hydroxide and answer each part
Please help with all parts 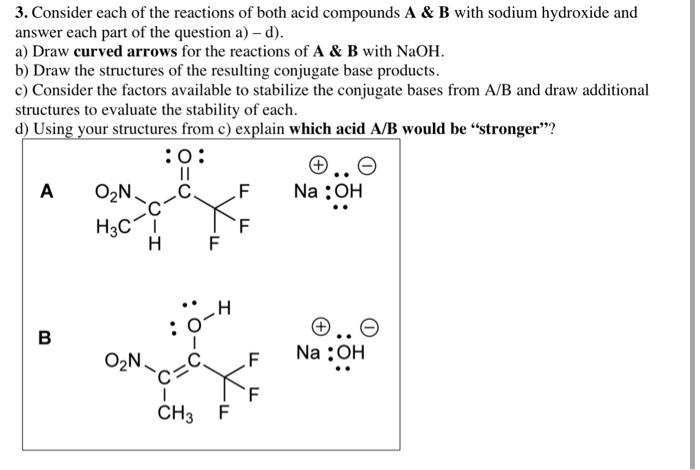
3. Consider each of the reactions of both acid compounds A & B with sodium hydroxide and answer each part of the question a) - d). a) Draw curved arrows for the reactions of A & B with NaOH. b) Draw the structures of the resulting conjugate base products. c) Consider the factors available to stabilize the conjugate bases from A/B and draw additional structures to evaluate the stability of each. d) Using your structures from c) explain which acid A/B would be stronger"? :0: A O2N. F Na : OH H3C1 H F O=O C C Y I I B ON Na : OH C=C Y f CH3 F 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


