Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with all parts if you can! Learning Goal: To use the Arrhenius equation to calculate the activation energy As temperature rises, the average
please help with all parts if you can! 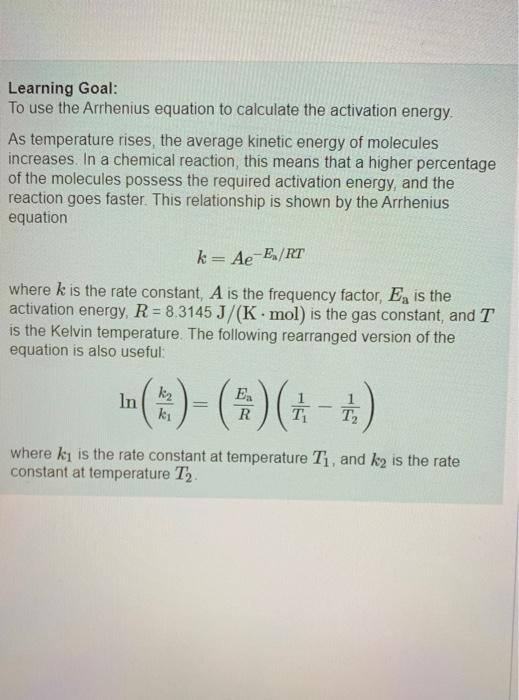
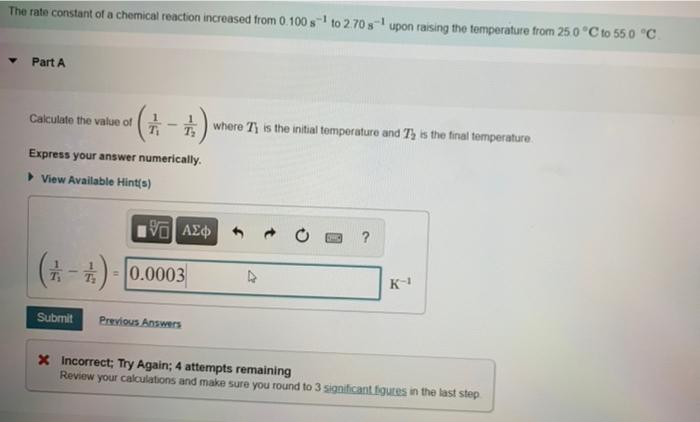
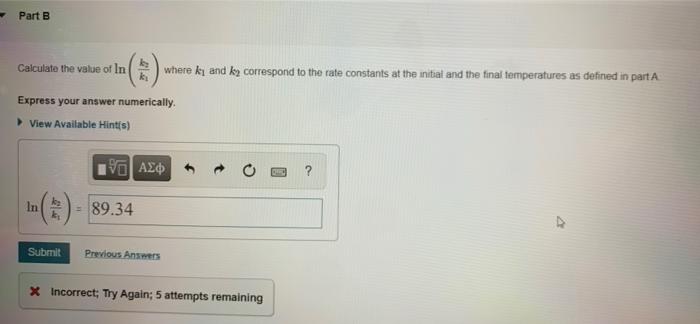
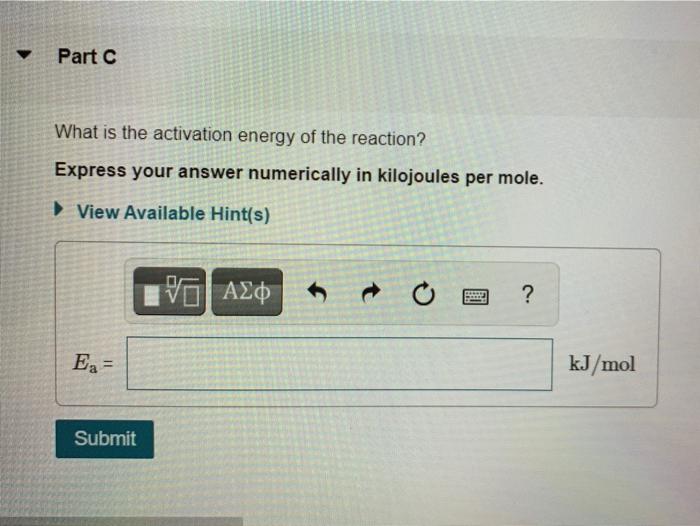
Learning Goal: To use the Arrhenius equation to calculate the activation energy As temperature rises, the average kinetic energy of molecules increases. In a chemical reaction, this means that a higher percentage of the molecules possess the required activation energy, and the reaction goes faster. This relationship is shown by the Arrhenius equation k= Ae-E/RT where k is the rate constant, A is the frequency factor, E, is the activation energy, R = 83145 J/(K.mol) is the gas constant, and T is the Kelvin temperature. The following rearranged version of the equation is also useful In I()-($)(+-+) . kz ki R T . T where ki is the rate constant at temperature T, and ky is the rate constant at temperature T2 The rate constant of a chemical reaction increased from 0.100 s- to 2.70 s upon raising the temperature from 250 C to 550 C Part A Calculate the value of (1-1) where Ty is the initial temperature and T, is the final temperature Express your answer numerically. View Available Hint(s) 19] (-) - 0.0003 K- Submit Previous Answers * Incorrect; Try Again; 4 attempts remaining Review your calculations and make sure you round to 3 significant figures in the last step Part B Calculate the value of In () where ki and ky correspond to the rate constants at the initial and the final temperatures as defined in part Express your answer numerically. View Available Hints) In 89.34 Submit Previous Answers X Incorrect; Try Again: 5 attempts remaining Part C What is the activation energy of the reaction? Express your answer numerically in kilojoules per mole. View Available Hint(s) VOI ? Ea= kJ/mol Submit 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


