Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with all three questions I am unsure of the steps for each of them! A 76.4g piete of metal at 69.0C is placed
please help with all three questions I am unsure of the steps for each of them! 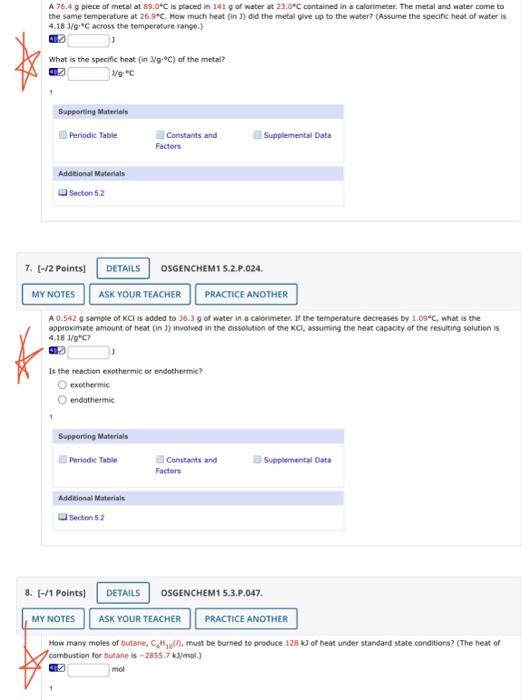
A 76.4g piete of metal at 69.0C is placed in 141g of water at 23.0C contained in a calorimeter. The metal and water come to the same temperature at 26.97C. How much heat (in 1) did the metal give up to the water? (Assume the specinc heat of water is 4.181/9C across the temperature range.) .] J What is the specific heat (in Jg+C) of the metal? [1] 1/9C Supporting Materiels Addelional Muterials [ Secton 5.2 [-/2 Points] OSGENCHEM1 5.2.P.024. A 0.542g sample of KCl is added to 36.3g of water in a calonimeter. If the temperature decreases by 1.09C, what is the approximate amount of heat (in J involved in the oissolution af the KCl, assuming the neat capacty of the resuiting solution is 4.181/9C ? J Is the reaction exothermic or endothermic? exothermic endothermic Supporting Materials Qillantants and Suplemental Dats Factors Addeiconat Materials 14 Secten 5.2 [-/1 Points] OSGENCHEM1 5,3,P,047. How many moles of butane, C4Hid[C, must be burned to produce 128k ) of heat under standard state conditions? (The heat of combustion for butane is 2855.7kJ mol.) [i] mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


