Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with both 2. A small amount of methanol (CH3OH) dissolved in cyclohexane (CcH12) boils at 100.57C. What is the solutions expected freezing point?
please help with both 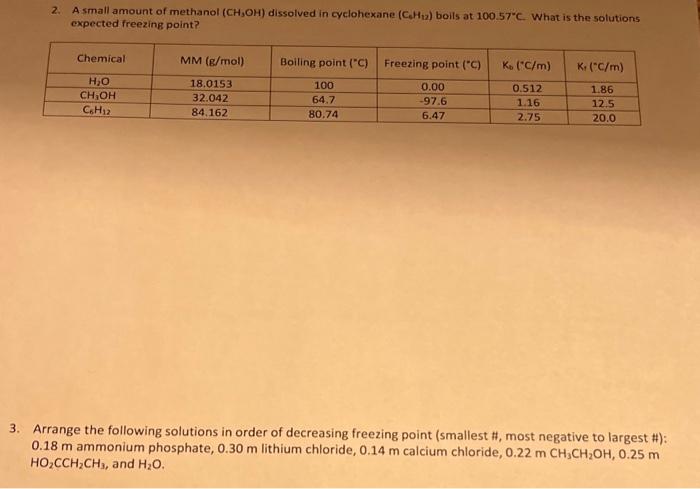
2. A small amount of methanol (CH3OH) dissolved in cyclohexane (CcH12) boils at 100.57C. What is the solutions expected freezing point? 3. Arrange the following solutions in order of decreasing freezing point (smallest #, most negative to largest \#): 0.18m ammonium phosphate, 0.30m lithium chloride, 0.14m calcium chloride, 0.22mCH3CH2OH,,2.25m HO2CCH2CH3, and H2O 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


