Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with both A student dissolves 15. g of styrene (C8H8) in 300 . mL of a solvent with a density of 1.20g/ml. The
please help with both 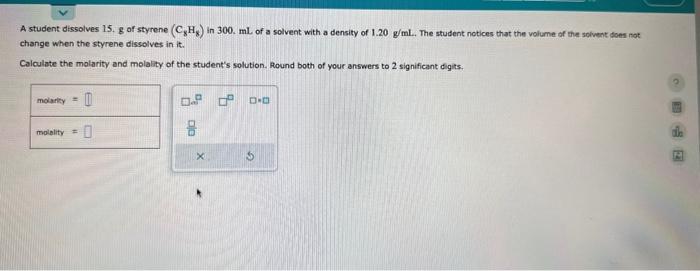
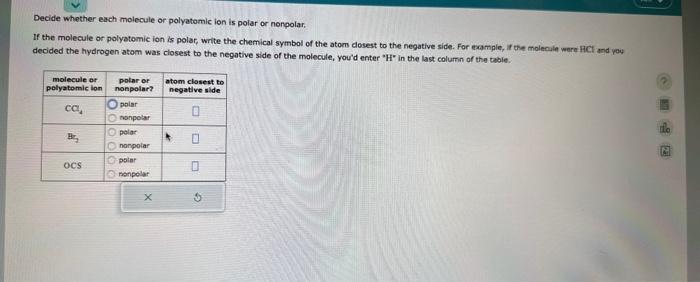
A student dissolves 15. g of styrene (C8H8) in 300 . mL of a solvent with a density of 1.20g/ml. The student notices that the volume of the solvent does not change when the styrene dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. Decide whether each molecule or polyatemic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom dosest to the negative side. For example, it the molecule ware HCl and yoy decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the tabie 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


