 please help with clear explanations.
please help with clear explanations.
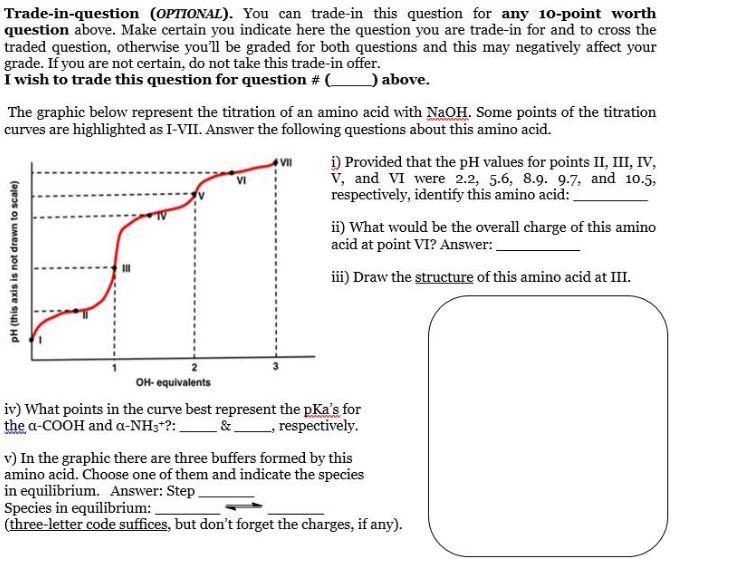
Trade-in-question (OPTIONAL). You can trade-in this question for any 10-point worth question above. Make certain you indicate here the question you are trade-in for and to cross the traded question, otherwise you'll be graded for both questions and this may negatively affect your grade. If you are not certain, do not take this trade-in offer. I wish to trade this question for question \# above. The graphic below represent the titration of an amino acid with NaOH. Some points of the titration curves are highlighted as I-VII. Answer the following questions about this amino acid. i) Provided that the pH values for points II, III, IV, V, and VI were 2.2,5.6,8.9.9.7, and 10.5, respectively, identify this amino acid: ii) What would be the overall charge of this amino acid at point VI? Answer: iii) Draw the structure of this amino acid at III. iv) What points in the curve best represent the pKa 's for the COOH and NH3+ ?: __ \& __ respectively. v) In the graphic there are three buffers formed by this amino acid. Choose one of them and indicate the species in equilibrium. Answer: Step Species in equilibrium: (three-letter code suffices, but don't forget the charges, if any). Trade-in-question (OPTIONAL). You can trade-in this question for any 10-point worth question above. Make certain you indicate here the question you are trade-in for and to cross the traded question, otherwise you'll be graded for both questions and this may negatively affect your grade. If you are not certain, do not take this trade-in offer. I wish to trade this question for question \# above. The graphic below represent the titration of an amino acid with NaOH. Some points of the titration curves are highlighted as I-VII. Answer the following questions about this amino acid. i) Provided that the pH values for points II, III, IV, V, and VI were 2.2,5.6,8.9.9.7, and 10.5, respectively, identify this amino acid: ii) What would be the overall charge of this amino acid at point VI? Answer: iii) Draw the structure of this amino acid at III. iv) What points in the curve best represent the pKa 's for the COOH and NH3+ ?: __ \& __ respectively. v) In the graphic there are three buffers formed by this amino acid. Choose one of them and indicate the species in equilibrium. Answer: Step Species in equilibrium: (three-letter code suffices, but don't forget the charges, if any)
 please help with clear explanations.
please help with clear explanations.





