Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with following questions given data attached in photo. Lab was to assay enzyme activity from B-galactaside and used sub subtrate o-nitrophenyl galactoside. Measured
please help with following questions given data attached in photo. Lab was to assay enzyme activity from B-galactaside and used sub subtrate o-nitrophenyl galactoside. Measured absorbance OD using spectrophotometer 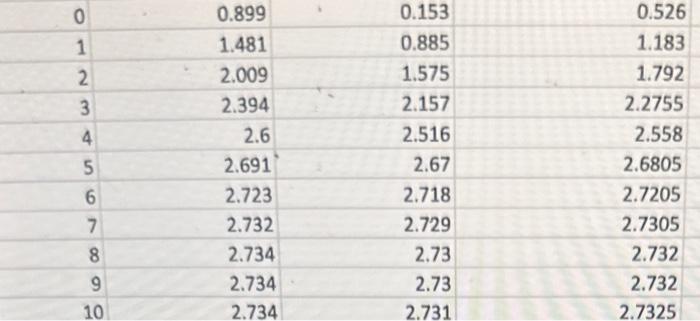
\begin{tabular}{|r|r|r|r|} \hline 0 & 0.899 & 0.153 & 0.526 \\ \hline 1 & 1.481 & 0.885 & 1.183 \\ \hline 2 & 2.009 & 1.575 & 1.792 \\ \hline 4 & 2.394 & 2.157 & 2.2755 \\ \hline 5 & 2.6 & 2.516 & 2.558 \\ \hline 6 & 2.691 & 2.67 & 2.6805 \\ \hline 7 & 2.723 & 2.718 & 2.7205 \\ \hline 8 & 2.732 & 2.729 & 2.7305 \\ \hline 9 & 2.734 & 2.73 & 2.732 \\ \hline 10 & 2.734 & 2.73 & 2.732 \\ \hline \end{tabular} \begin{tabular}{|r|r|r|r|} \hline 0 & 0.899 & 0.153 & 0.526 \\ \hline 1 & 1.481 & 0.885 & 1.183 \\ \hline 2 & 2.009 & 1.575 & 1.792 \\ \hline 4 & 2.394 & 2.157 & 2.2755 \\ \hline 5 & 2.6 & 2.516 & 2.558 \\ \hline 6 & 2.691 & 2.67 & 2.6805 \\ \hline 7 & 2.723 & 2.718 & 2.7205 \\ \hline 8 & 2.732 & 2.729 & 2.7305 \\ \hline 9 & 2.734 & 2.73 & 2.732 \\ \hline 10 & 2.734 & 2.73 & 2.732 \\ \hline \end{tabular} Question: Use the extinction coefficient for ONP to calculate the initial concentration in moles/L (Molar). Extiniction coefficient of ONP --> 2.5x10^-3 M ONP has an optical density of 1.0/minute.
question: calculate reaction rates for intervals given table.
data:

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


