Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with part b. I added some notes that might help identify the halide Experiment #12: Electrochemical Cell Potential and the Activity Series Report
please help with part b. I added some notes that might help identify the halide 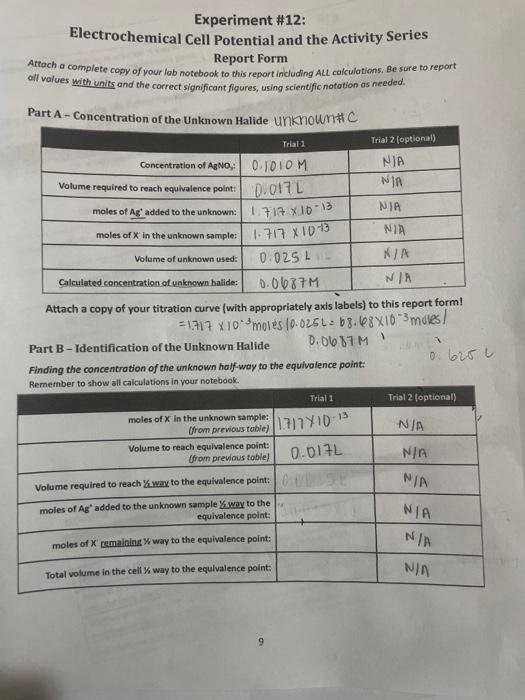
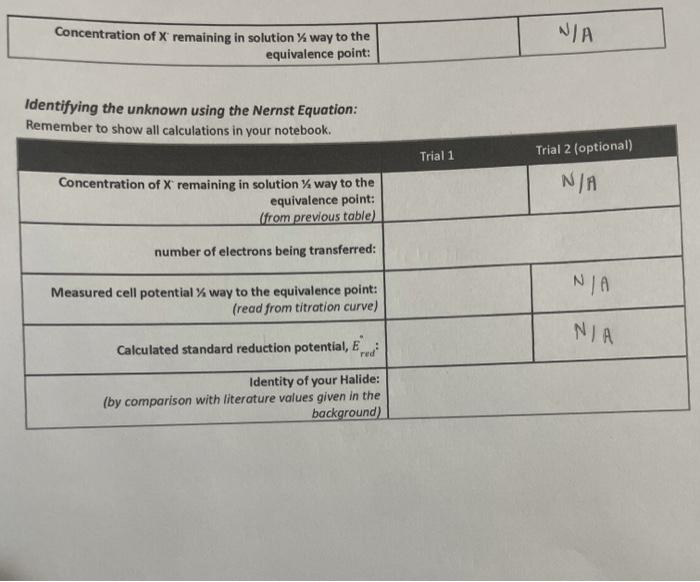
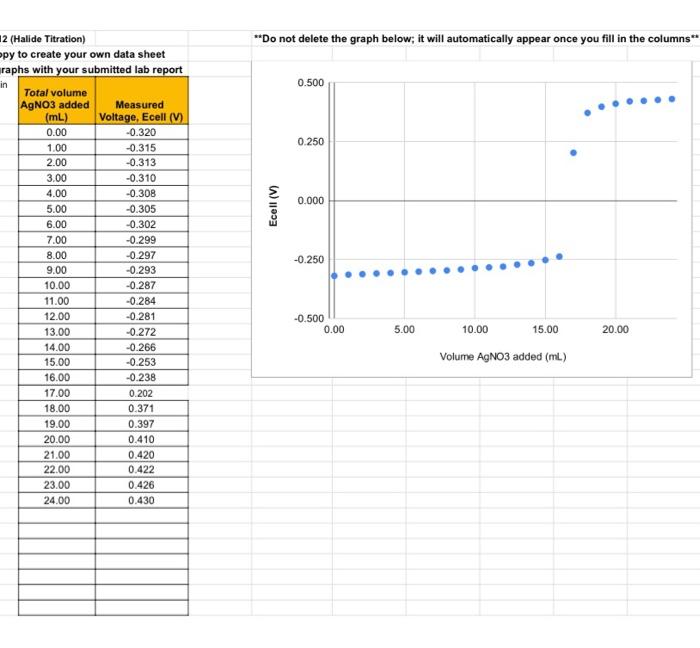

Experiment \#12: Electrochemical Cell Potential and the Activity Series Report Form Attach a complete capy of your lab notebook to this report including ALl cakulations. Be sure to report all values with units and the correct significant figures, using scientific nototion as needed. Part A - Concentration of the Unknown Halide Unknownt C Attach a copy of your titration curve (with appropriately axis labels) to this report form! =1.717103moles10.025L=68.48103 mules 1 Part B - Identification of the Unknown Halide Finding the concentration of the unknown half-way to the equivalence point: Dmmanker tn chnw all calculations in vour notebook. Identifying the unknown using the Nernst Equation: Remember to show all calculations in your notebook. 2 (Halide I Itrationi I The next step in the process is to identify the unknown halide. To do this, it is important to understand that the reaction occurring at the cathode is different than the titration reaction. The titration reaction is a precipitation reaction while the cathode reaction is actually a reduction half reaction. For this experiment, there are three possible unknowns (Cl,Br, and l) with the following corresponding reduction half-reactions occurring at the cathode: AgCl(n)+eAg(n)+C(aq)E(aithad=0.222VAgBr(v)+eAg(0)+Br(+9)Ecaihad=0.0713VAgI(0)+eAg(x)+I(ae)E(athotr=0.151V Notice that the reaction at the cathode depends on the product of the titration reaction. By comparing the literature value shown here with a calculated standard reduction potential, it is possible to distinguish between the three halides. The reaction quotient, Q, for these half-reactions can be written as: Q=[x]Eqn.2 where [X] is the concentration of the halide in solution, Remember that the solids drop out of the reaction quotient. Since the reaction is occurring at the cathode, we use a standard reference electrode for the anode, which has a constant half-cell potential (in this case of 0.197V ). This isolates just the reaction at the cathode. By combining the Nernst equation with the equation for cell potential the overall electrochemical cell can be expressed: Eceil=EcathadeEanodeEcell=[Ecathode0.05916loglog(X)]0.197EqEq.4 Identifying the unknown can be done by matching the calculatedstandard electrode potential using a measured cell potential at a known (or measured) concentration to known values for the same half-reaction. This must be done using a measured cell potential and the concentration of halide remaining in the flat region between 3070% of the way to the equivalence point. The calculated standard cell potential from the sample should be very close to one of the three listed values, allowing the identity of the unknown halide to be determined 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


