Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with post lab questions Experimental Data and Results Part AL Exothermic and Endothermie Dissolution of Salts Naci CaCl2 KCI Equation for Dissolution of
please help with post lab questions 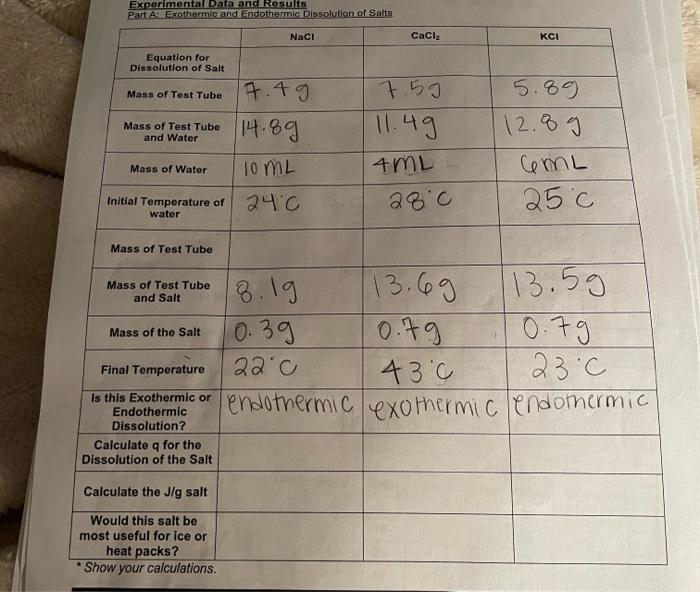
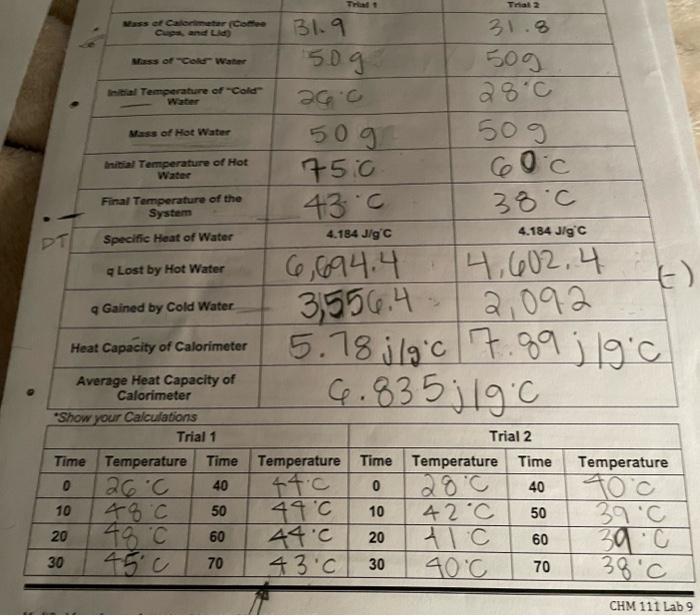
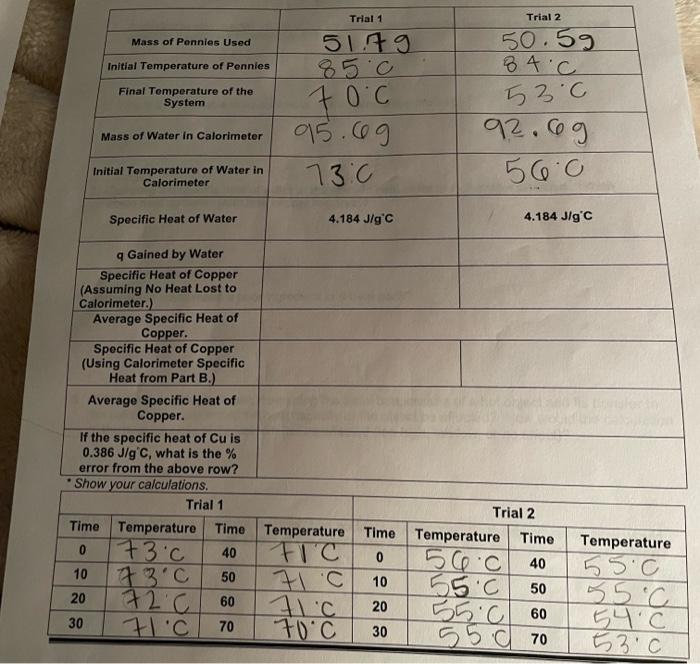

Experimental Data and Results Part AL Exothermic and Endothermie Dissolution of Salts Naci CaCl2 KCI Equation for Dissolution of Salt Mass of Test Tube 750 Mass of Test Tube and Water 7.49 14.89 10ML 11.49 5.89 12.89 ceML 25c Mass of Water 4ML 28.0 Initial Temperature of water 24C Mass of Test Tube Mass of Test Tube and Salt Mass of the Salt 18.19 (13.69 113.5g 0.39 0.79 10.79 22c 43c 23.0 is the best teremte or endothermic exothermic endothermic Final Temperature Is this Exothermic Endothermic Dissolution? Calculate q for the Dissolution of the Salt Calculate the J/g salt Would this salt be most useful for ice or heat packs? Show your calculations. Trial 2 ass et Cantar (Come Cand Lid 31.9 Mass of Coke Water sog 31.8 50g 28c al Temperature of Cold acc Mas of Hot Water 50g 50g Initial Temperature of Hot Water 75.0 43c 60c 38.c Final Temperature of the System Specific Heat of Water PT 4.184 Jig C 4.184 Jig C Lost by Hot Water 6,694.4 14,602.4 3,556.4 2,092 1) Gained by Cold Water Heat Capacity of Calorimeter 5.78j|g'c 7.89 ; 19 cl 6.835; 19C Average Heat Capacity of Calorimeter "Show your Calculations Trial 1 Time Temperature Time 0 26C 40 10 48C 50 48C 60 30 70 Trial 2 Temperature Time Temperature Time 44.0 0 280 40 10 42C 50 44.0 20 41c 60 43.0 40C 70 Temperature 39C 49C 20 888 30 38C CHM 111 Lab 9 Trial 1 Trial 2 Mass of Pennies Used 51.79 50.59 84.C Initial Temperature of Pennies Final Temperature of the System 70c 53c Mass of Water in Calorimeter 95.09 92. og 56.0 Initial Temperature of Water in Calorimeter 73.0 Specific Heat of Water 4.184 J/g C 4.184 J/g'c 9 Gained by Water Specific Heat of Copper (Assuming No Heat Lost to Calorimeter.) Average Specific Heat of Copper. Specific Heat of Copper (Using Calorimeter Specific Heat from Part B.) Average Specific Heat of Copper. If the specific heat of Cu is 0.386 J/g C, what is the error from the above row? Show your calculations. Trial 1 Trial 2 Time Temperature Time Temperature Time Temperature Time 0 73c 40 FIC 0 56.c 40 10 23c 50 7.C 10 155.0 50 20 72.C 60 TIC 20 55.0 60 30 7.C 70 70C 30 5570 Temperature 55.0 55C 54C 53.0 1. According to your results, what salt in Part A would have been the best choice for use in a heat pack? In a cold pack? Defend your choice 2. Why is it not possible to reuse a heat pack in term of the chemistry? 3. Compare the specific heat of water to the specific heat of metal in the table provided. Which would heat up faster (with less energy required)? 4. Why would metal make a poor ingredient in a heat pack? 5. If there was a delay between measuring the initial temperature of a hot object and its transfer to the calorimeter, how would the heat capacity of the object be affected? How would the calculation of the heat capacity of the calorimeter be affected (too high, too low, or no affect)? 6. If hot water from the test tube in Part C had accidentally dripped into the calorimeter, how would the calculation of the specific heat of the calorimeter be affected (too high, too low, or no affect) 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


