Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with problem 1 with the given information The table below shows what happens to the concentration of phenolphthalein in a solution that was
please help with problem 1 with the given information 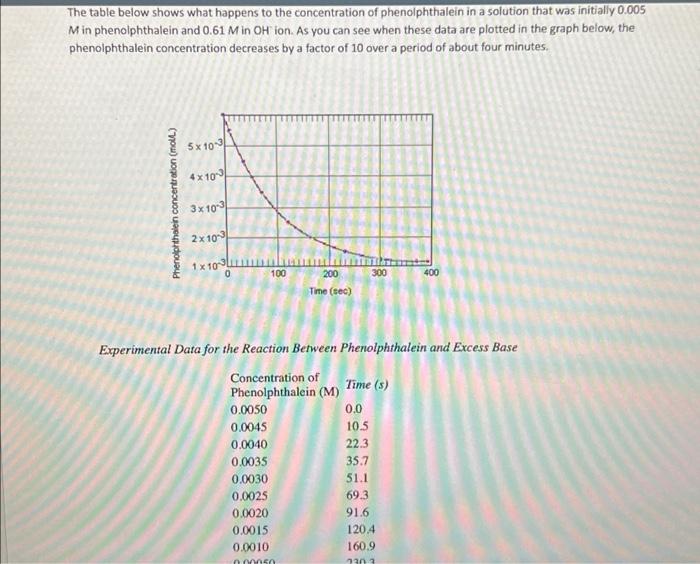
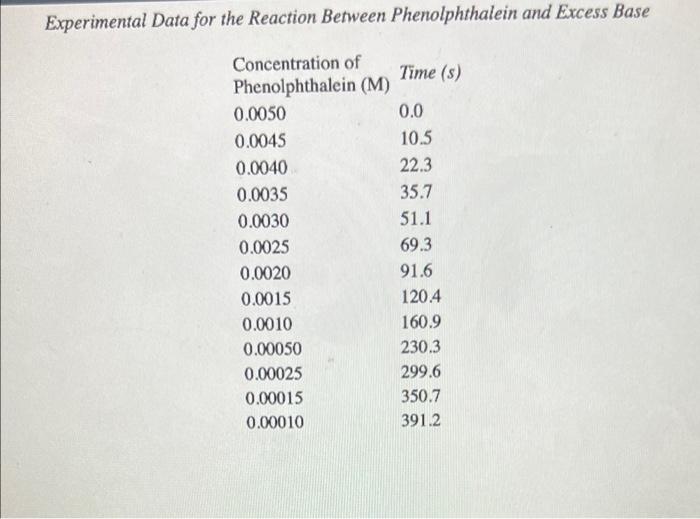

The table below shows what happens to the concentration of phenolphthalein in a solution that was initially 0.005 Min phenolphthalein and 0.61 Min Oh'ion. As you can see when these data are plotted in the graph below, the phenolphthalein concentration decreases by a factor of 10 over a period of about four minutes, 5x100 4x100 Phenolphthalein concentration (moll) 3x 100 2x 100 1x10314 100 300 400 200 Time (sec) Experimental Data for the Reaction Between Phenolphthalein and Excess Base Concentration of Phenolphthalein (M) Time (8) 0.0050 0.0 0.0045 10.5 0.0040 22.3 0.0035 35.7 0.0030 51.1 0.0025 69.3 0.0020 91.6 0.0015 1204 0.0010 160.9 Experimental Data for the Reaction Between Phenolphthalein and Excess Base Concentration of Time (s) Phenolphthalein (M) 0.0050 0.0 0.0045 10.5 0.0040 22.3 0.0035 35.7 0.0030 51.1 0.0025 69.3 0.0020 91.6 0.0015 120.4 0.0010 160.9 0.00050 230.3 0.00025 299.6 0.00015 350.7 0.00010 391.2 Problem 1: Use the data in the above table to calculate the rate at which phenolphthalein reacts with the OH' ion during each of the following periods: (a) During the first time interval, when the phenolphthalein concentration falls from 0.0050 M to 0.0045 M. (b) During the second interval, when the concentration falls from 0.0045 M to 0.0040 M. (c) During the third interval, when the concentration falls from 0.0040 M to 0.0035 M 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


