Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with section IV and Section V sorry here's the graph can you please let me know what exactly you need? not clear to
please help with section IV and Section V 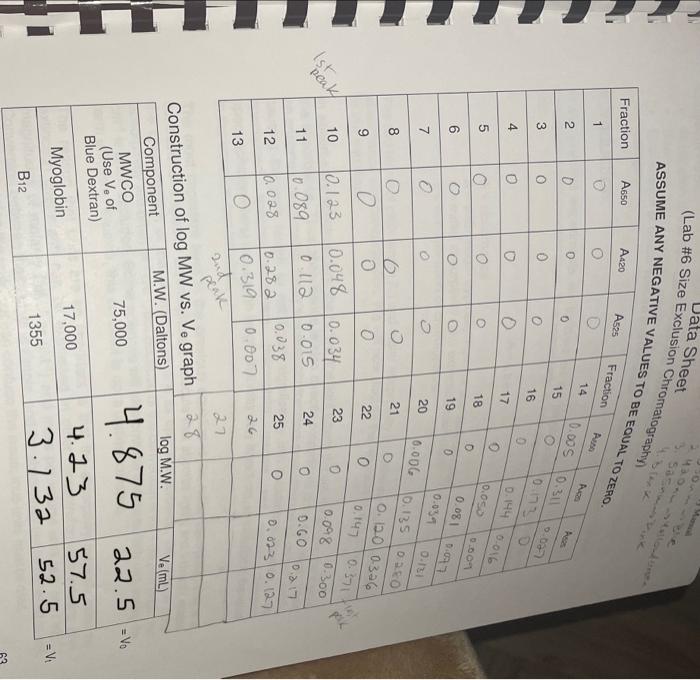





sorry here's the graph 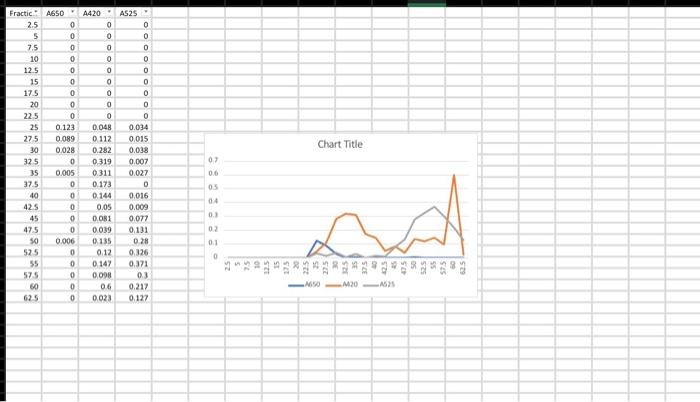

can you please let me know what exactly you need?
not clear to understand? all the data points are there and fractions 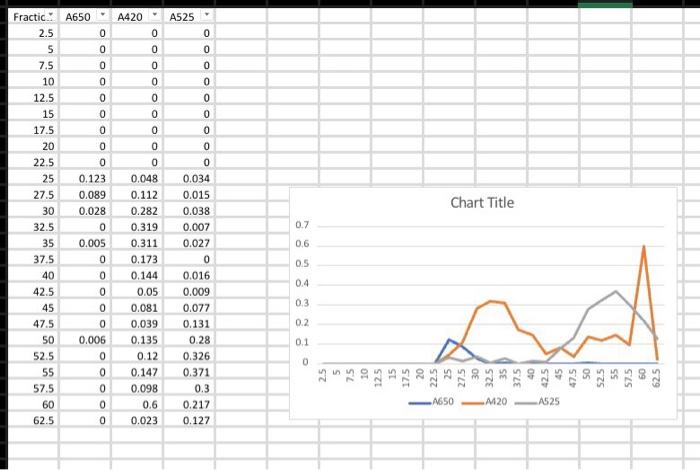

please let me know If this is enough 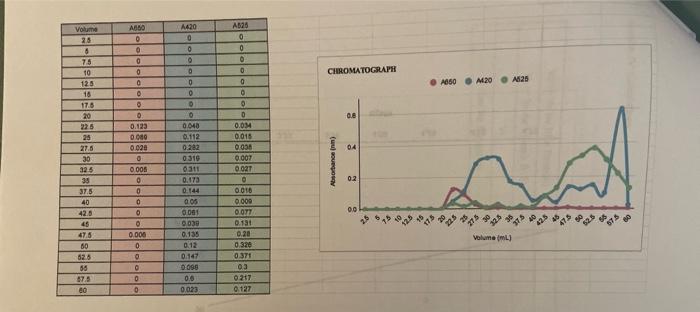

what do you need?? please specify
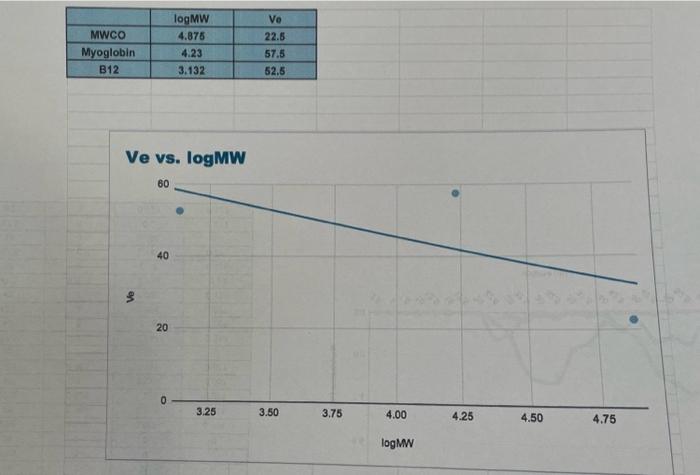
(Lab \#6 Size Exta Sheet ASSUME ANY NEGAT... Report Instructions: Section 1: Data Sheet (Sheet from Book) - Fill in all necessary absorbance data. - In the second table, pertorm a logarithm of the given molecular weights and enter it under "log MW". Fill in the appropriate Ve for each component (you will need to construct the chromatograph before completing this) Section II: Chromatograph (1 page) - Using either Excel or graph paper, plot Absorbance vs. Volume tor each wavelength. Remember, we are using 2.5mL fractions, so you wili have to convert each traction number into a volume. The dots for each curve should be connected, and each wavelength should have its own curve but still all on the same graph. DO NOT perform any type of curve fit. - Identify on the graph which peak corresponds to which component and what their elution volumes (V0) are. As diagramed in Figure 6.2, elution from the column is nol sharp but can spread out over a number of samples. Vo is defined as the midpoint of the peak, which is often the highest part of the peak. Section III: Ve vs. Log MW (1 page) - Using the data from the second table on the data sheet, construct a graph of Ve vs. log MW. This should be a scatter plot constructed on either graph paper or in Excel. Perform a best-line fit (by hand or by Excel) on these three points. Section IV: Calculations (1 page or less) - In tabular form, please type the following: V0(=V0 for Blue Dextran) Vi(=Ve for Vitamin B12) Vi Kav for Myoglobin - Provide calculations for Vi and Kav - Answer the following questions with TYPED responses. Handwritten respons Section V: Questions ( 12 pages total) 1. You are looking to separate a solution containing two proteins: one at 15kDa WILL NOT BE ACCEPTED. one at 50kDa. Which would be the best MWCO to use: G-25, G-50, or G-1 Why? 2. Using the graph you constructed in Section III, determine the approximate Ve for each of the following proteins. Show the data in tabular form. (NOTE: The molecular I.ninhta aro niven in kiloDaltons, not Daltons). In what order would the proteins elute from a G-75 column? Using the Kav you calculated for Myoglobin, calculate what the Ve would be for Myoglobin would be on a G-75 column with a V0 of 25mL and a Vt of 120mL. V0=2SmLVt=120mLVi=95 \begin{tabular}{|c|c|c|} \hline & logMW & Ve \\ \hline MWCO & 4.875 & 22.5 \\ \hline Myoglobin & 4.23 & 57.5 \\ \hline B12 & 3.132 & 52.5 \\ \hline \end{tabular} Ve vs. logMW 6040 $ 20 03.253.503.75logMN4.004.254.504.75 (Lab \#6 Size Exta Sheet ASSUME ANY NEGAT... Report Instructions: Section 1: Data Sheet (Sheet from Book) - Fill in all necessary absorbance data. - In the second table, pertorm a logarithm of the given molecular weights and enter it under "log MW". Fill in the appropriate Ve for each component (you will need to construct the chromatograph before completing this) Section II: Chromatograph (1 page) - Using either Excel or graph paper, plot Absorbance vs. Volume tor each wavelength. Remember, we are using 2.5mL fractions, so you wili have to convert each traction number into a volume. The dots for each curve should be connected, and each wavelength should have its own curve but still all on the same graph. DO NOT perform any type of curve fit. - Identify on the graph which peak corresponds to which component and what their elution volumes (V0) are. As diagramed in Figure 6.2, elution from the column is nol sharp but can spread out over a number of samples. Vo is defined as the midpoint of the peak, which is often the highest part of the peak. Section III: Ve vs. Log MW (1 page) - Using the data from the second table on the data sheet, construct a graph of Ve vs. log MW. This should be a scatter plot constructed on either graph paper or in Excel. Perform a best-line fit (by hand or by Excel) on these three points. Section IV: Calculations (1 page or less) - In tabular form, please type the following: V0(=V0 for Blue Dextran) Vi(=Ve for Vitamin B12) Vi Kav for Myoglobin - Provide calculations for Vi and Kav - Answer the following questions with TYPED responses. Handwritten respons Section V: Questions ( 12 pages total) 1. You are looking to separate a solution containing two proteins: one at 15kDa WILL NOT BE ACCEPTED. one at 50kDa. Which would be the best MWCO to use: G-25, G-50, or G-1 Why? 2. Using the graph you constructed in Section III, determine the approximate Ve for each of the following proteins. Show the data in tabular form. (NOTE: The molecular I.ninhta aro niven in kiloDaltons, not Daltons). In what order would the proteins elute from a G-75 column? Using the Kav you calculated for Myoglobin, calculate what the Ve would be for Myoglobin would be on a G-75 column with a V0 of 25mL and a Vt of 120mL. V0=2SmLVt=120mLVi=95 \begin{tabular}{|c|c|c|} \hline & logMW & Ve \\ \hline MWCO & 4.875 & 22.5 \\ \hline Myoglobin & 4.23 & 57.5 \\ \hline B12 & 3.132 & 52.5 \\ \hline \end{tabular} Ve vs. logMW 6040 $ 20 03.253.503.75logMN4.004.254.504.75 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


