Question: Please help with solution concentrations. I'm working on prelab and need help with the concentrations. Also, the chemical structure would be appreciated along with an


Please help with solution concentrations. I'm working on prelab and need help with the concentrations. Also, the chemical structure would be appreciated along with an explanation.
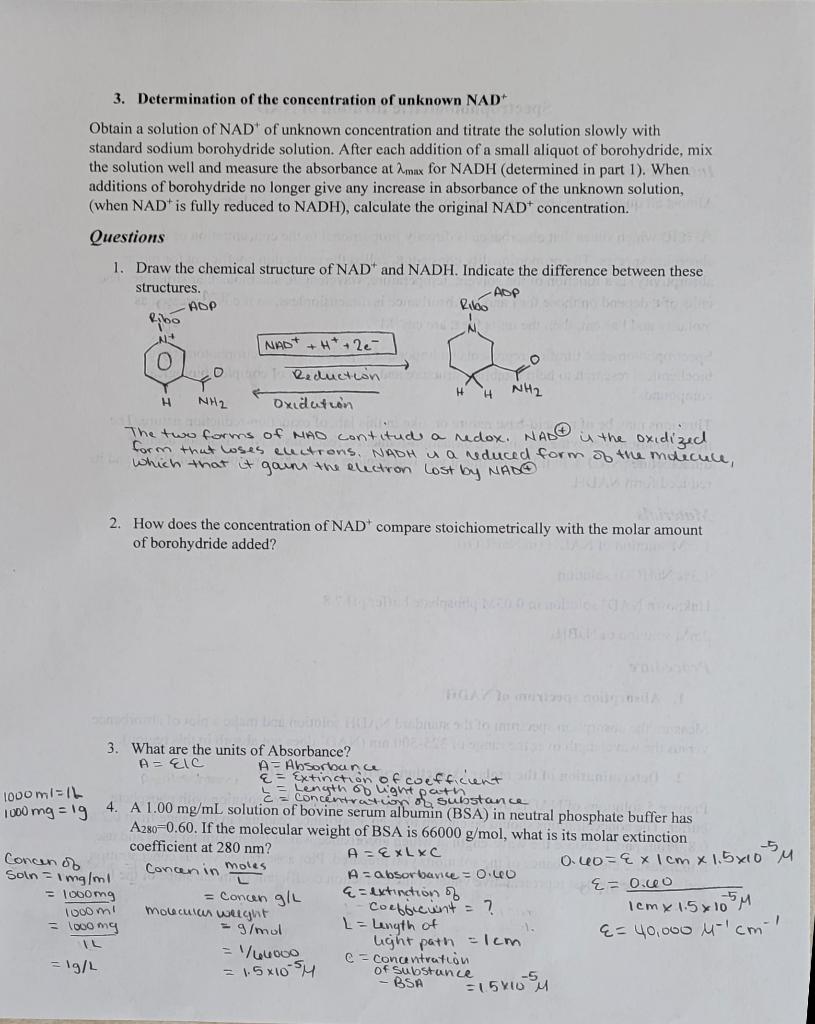
5. The concentrations of five solutions of cytidine at pH-7, along with their representative absorbance values at 271 nm are given below. Is Beer's law obeyed within experimental error? Include the graph. c (mg/L) au points are connected in a strought line 0.684 18.30 Palues through the orgin 0473 12.65 Yes. Beers law is obeyed 0.296 7.92 -Graph attached 0.135 3.61 0.097 2.59 Greiph 6. Part of setting up a spectrophotometric assay is choosing the proper wavelength of observation. For phosphate determination, the standard solutions were run using two different wavelengths with the following results: attached C (phosphate M) 0 0.2 0.4 0.7 LO Absorbance at 21 0 0.09 0.18 0.29 0.42 Absorbance at 22 0 0.05 0.10 0.16 0.23 Which would be the better wavelength for the determination? Explain. See graph 7. What is the mathematical relationship between percent transmittance and absorbance? A= 2 - log (9.T) % - 20% A = 2-log 20 - 2-1.301 Calculate: -0.699 20% T- A=0.7 Attach the graphs, data table, all your calculations and a short conclusion to your lab report. 3. Determination of the concentration of unknown NAD Obtain a solution of NAD of unknown concentration and titrate the solution slowly with standard sodium borohydride solution. After each addition of a small aliquot of borohydride, mix the solution well and measure the absorbance at max for NADH (determined in part 1). When additions of borohydride no longer give any increase in absorbance of the unknown solution, (when NAD is fully reduced to NADH), calculate the original NAD concentration. Questions 1. Draw the chemical structure of NAD and NADH. Indicate the difference between these structures. -AOP ADP Ribo Ribo INAD+ + H+ + 2e- N NE NH2 Reduction # NH2 Oxidation The two forms of WAO contitud a redox. NAD is the oxidized form that loses electrons. NADH is a reduced form of the molecule, ich that it goins the electron Lost by NADA 2. How does the concentration of NAD compare stoichiometrically with the molar amount of borohydride added? 1000 mia IL 3. What are the units of Absorbance? A = CIC A=Absorbance e Extinction of coefficient = Concentrado o substance = length of light paten 1000 mg = 1g 4. A 1.00 mg/ml solution of bovine serum albumin (BSA) in neutral phosphate buffer has A280=0.60. If the molecular weight of BSA is 66000 g/mol, what is its molar extinction coefficient at 280 nm? Concen of A-ExLxc 0.40=Ex 1cm x 1.5x10 M Concen in moles A borbavice = O.LV E=0.60 = 100mg = concen g/L &= extinction of Molecular weight Coefficiunt = ? loomy - g/mol L = Length of &= 40,000 M-1cm1 IL = 1/60000 light path = 1cm = 1g/L = 1.5610 SM C- Substance BSA 2 15x105 m Soln = 1 mg/ml 1cm x 15 x 105M looo mi Spectrophotometric titration of NAD+ c Introduction Almost all quantitative absorption spectrophotometry is based on Beer's law: A=elc which states that absorbance is directly proportional to the concentration of the absorbing species. The proportionality constante, called the extinction coefficient (or molar absorptivity) is a function of the solvent, temperature, wavelength, and molecular structure. The units of depend on those for I and c. Absorbance is dimensionless, so if c is expressed as molarity and I as cm, then the units for are cm 'M". Spectrophotometric titration represents an important application of spectrophotometry in biochemical research to help establish structure and concentration of complex biological compounds. Titrations may be of an acid-base nature or, like in this lab, of oxidation-reduction nature. The most common coenzyme required in oxidation-reduction reactions is Nicotinamide adenine dinucleotide (NAD'). In this experiment you will explore some of the properties of NAD and its reduced form NADH. Materials 1 mM solution of NADH (in NaHCO3) 1.3% NaHCO3 solution Unknown NAD solution in 0.05M phosphate buffer pH 7.8 2mM solution of NaBHA Procedure 1. Absorption spectrum of NADH Measure the absorption spectrum of the standard NADH solution and make a plot of absorbance versus the wavelength over the range of 325-500 nm (NAD' does not absorb in this region). 2. Determination of the molar extinction coefficient of NADH Prepare 5 solutions of NADH by appropriate dilution of the standard solution with phosphate buffer. The concentrations should be chosen to keep absorbance readings in the range from 0.2 to 0.8. From part 1 choose the wavelength at which absorbance was at the maximum level and use it to measure the absorbance of the solutions you prepared. Plot absorbance versus concentration and calculate the best representative value of the extinction coefficient from the slope of the line (1=1cm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


