Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with the Q2 . The table is for Q2 Q1 (a) The work done on an elevated body is due to the weight
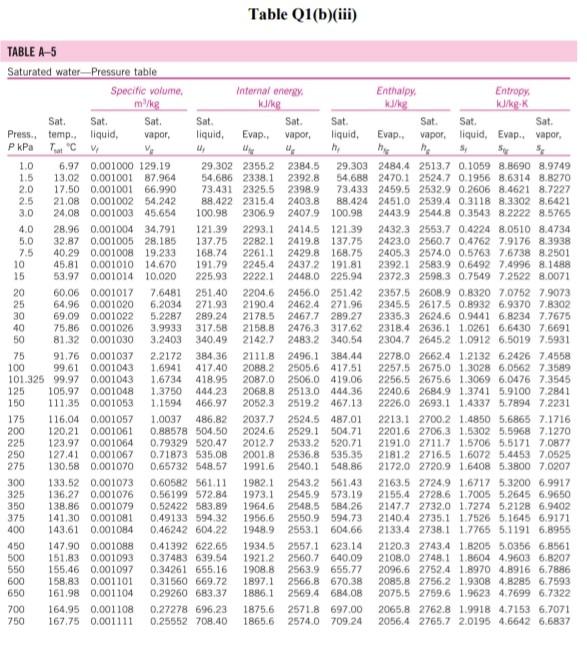
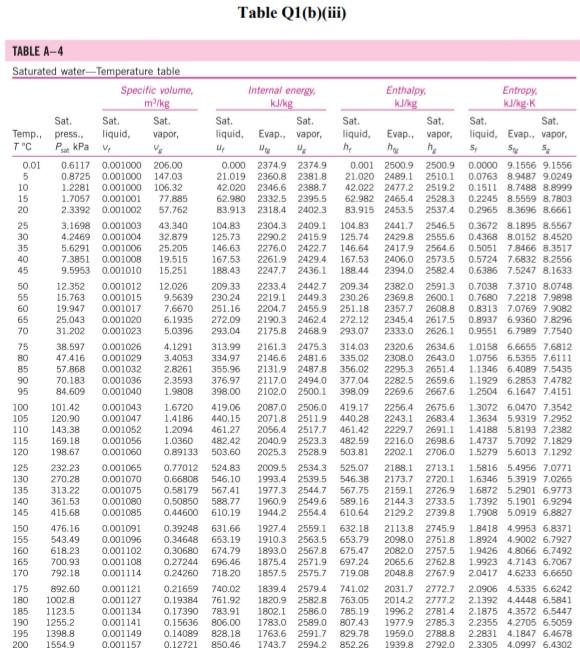
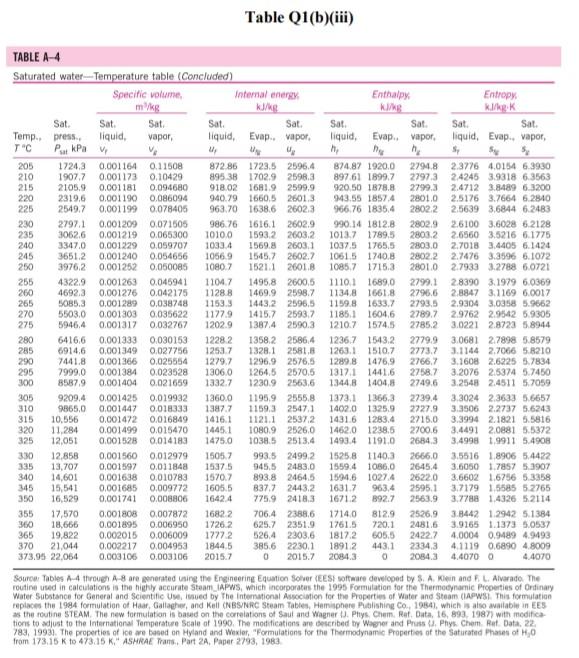
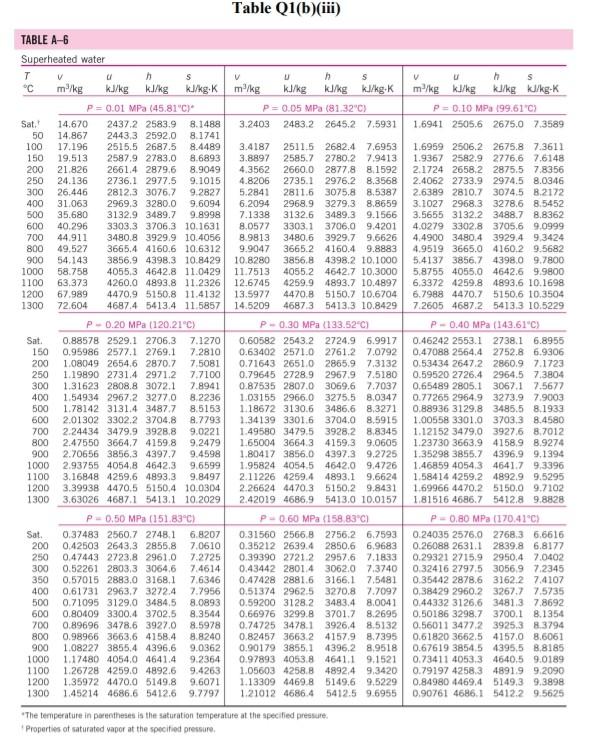
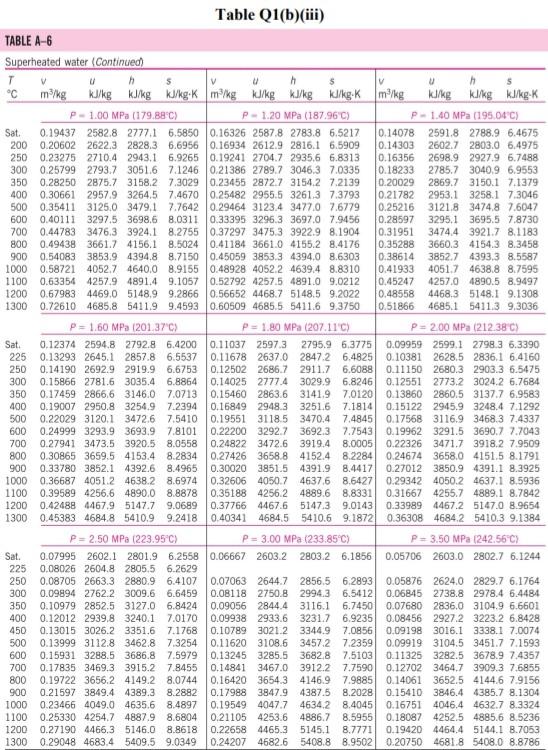
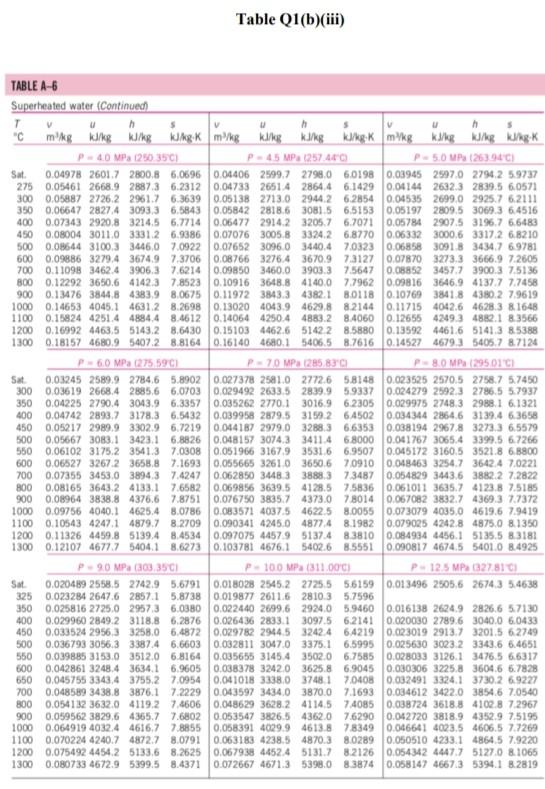
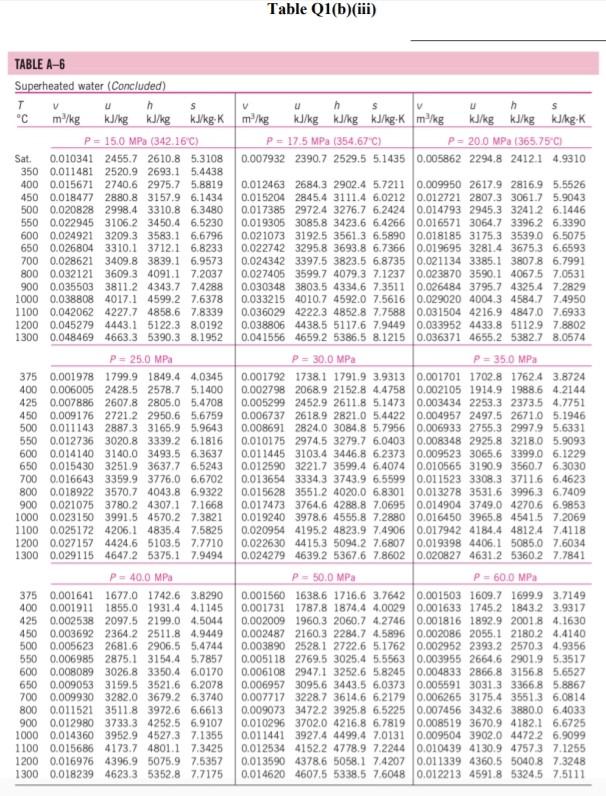

Please help with the Q2. The table is for Q2
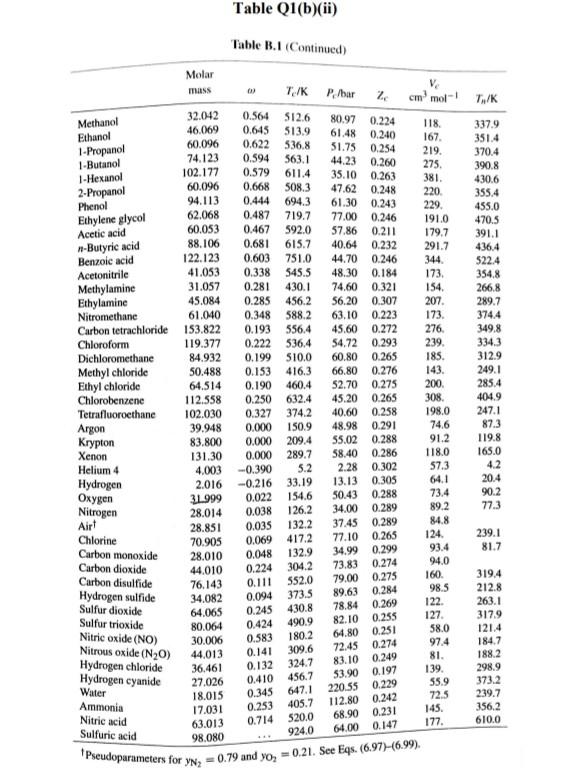
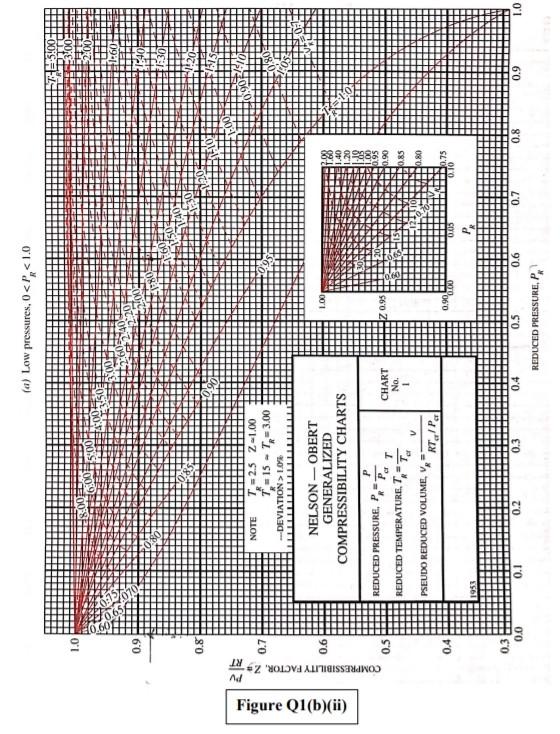
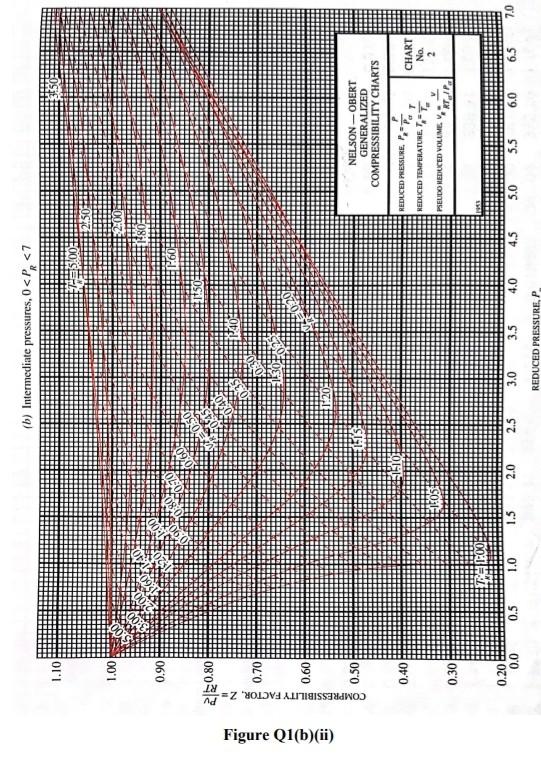
Q1 (a) The work done on an elevated body is due to the weight of a body. Express the potential energy based on abovementioned body with respect to elevation i.e. the distance. (4 marks) (b) Explain isobaric, isochoric and polytropic processes for an ideal gas. When explaining these processes, draw a pressure-volume (P-V) diagram for each process, as well as the path function. (5 marks) (c) Polytropic process of an ideal gas can be expressed either in P-V or T-V relationships. Show that a work which is shown in equation below can be from 8w = PdV (include your assumptions): wv = P.V. [L ()] in 1 (8 marks) (d) Distinguish between above work and the below work, as shown in equations Q1 (e) and Q1 (d) respectively. Note: First, list steps to obtain below work, which can be derived from 8w = s PDV: P2V2 - PV W= 1-n (8 marks) Table Q1(b)(ii) Table B.1 (Continued) 2 cm mol- T./K 337.9 351.4 370.4 390.8 430.6 275 355.4 229 173. Molar mass T/K P/har 32.042 0.564 Methanol $12.6 80.97 0.224 46.069 0.645 118. Ethanol 513.9 61.48 0.240 60.096 1-Propanol 167 0.622 536.8 51.75 0.254 74.123 0.594 219. 1-Butanol 563.1 44.23 0.260 1-Hexanol 102.177 0.579 611.4 35.10 0.263 60.096 381 0.668 2-Propanol 508.3 47.62 0.248 220 Phenol 94.113 0.444 694.3 61.30 0.243 Ethylene glycol 62.068 0.487 719.7 77.00 0.246 191.0 Acetic acid 60.053 0.467 592.0 57.86 0.211 179.7 --Butyric acid 88.106 0.681 615.7 40.64 0.232 291.7 Benzoic acid 122.123 0.603 751.0 44.70 0.246 344 Acetonitrile 41.053 0.338 545.5 48.30 0.184 173 Methylamine 31.057 0.281 430.1 74.60 0.321 154. Ethylamine 45.084 0.285 456.2 56.20 0.307 207 Nitromethane 61.040 0.348 588.2 63.10 0.223 Carbon tetrachloride 153.822 0.193 556.4 45.60 0.272 276 Chloroform 119.377 0.222 536.4 54.72 0.293 239. Dichloromethane 84.932 0.199 510.0 60.80 0.265 185. Methyl chloride 50.488 0.153 416.3 66.80 0.276 143 Ethyl chloride 64.514 0.190 460.4 52.70 0.275 200 Chlorobenzene 112.558 0.250 632.4 45.20 0.265 308 Tetrafluoroethane 102.030 0.327 374.2 40.60 0.258 198.0 Argon 39.948 0.000 150.9 48.98 0.291 74.6 Krypton 83.800 0.000 209.4 55.02 0.288 91.2 Xenon 131.30 0.000 289.7 58.40 0.286 118.0 Helium 4 4.003 -0.390 5.2 2.28 0.302 57.3 Hydrogen 2.016 -0.216 33.19 13.13 0.305 64.1 Oxygen 3.1999 0.022 154.6 50.43 0.288 73.4 Nitrogen 28.014 0.038 126.2 34.00 0.289 89.2 Airt 28.851 0.035 132.2 37.45 0.289 84.8 Chlorine 70.905 0.069 417.2 77.10 0.265 124 Carbon monoxide 0.048 28.010 132.9 34.99 93.4 0.299 Carbon dioxide 44.010 0.224 304.2 73.83 94.0 0.274 Carbon disulfide 76.143 160 0.111 552.0 79.00 0.275 Hydrogen sulfide 34,082 0.094 373.5 89.63 0.284 98.5 Sulfur dioxide 64.065 122 0.245 78.84 430.8 0.269 Sulfur trioxide 127 80.064 0.424 0.255 490.9 82.10 Nitric oxide (NO) 30.006 0.583 180.2 58.0 64.80 0.251 Nitrous oxide (N20) 0.274 97.4 44,013 72.45 0.141 309.6 Hydrogen chloride 0.249 81. 36.461 0.132 83.10 324.7 Hydrogen cyanide 139. 27.026 0.410 53.90 0.197 456.7 Water 55.9 0.229 18,015 0.345 220.55 647.1 Ammonia 72.5 17.031 0.253 405.7 112.80 0.242 Nitric acid 0.231 145. 68.90 520.0 0.714 63.013 Sulfuric acid 177. 64.00 0.147 924.0 98.080 Pseudoparameters for yN2 =0.79 and yo, = 0.21. See Eqs. (6.97)-(6.99). 455.0 470.5 391.1 4364 522.4 354.8 266.8 289.7 374.4 349.8 334.3 312.9 249.1 285.4 404.9 247.1 87.3 119.8 165.0 4.2 20.4 90.2 77.3 239.1 81.7 319.4 212.8 263.1 317.9 121.4 184.7 188.2 298.9 373.2 239.7 356.2 610.0 0P (a) Low pressures, 0Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


