Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with these post lab questions (7 pts, 1 pt each no partial credit) In your lab report answer the following questions and briefly
please help with these post lab questions 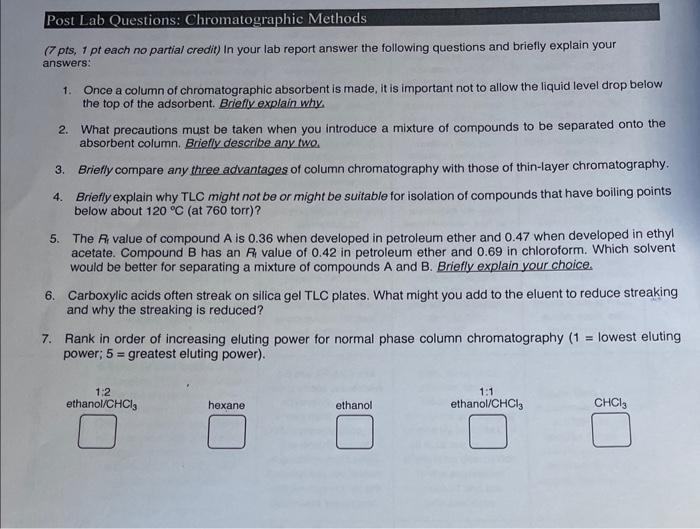
(7 pts, 1 pt each no partial credit) In your lab report answer the following questions and briefly explain your answers: 1. Once a column of chromatographic absorbent is made, it is important not to allow the liquid level drop below the top of the adsorbent. Briefly explain why. 2. What precautions must be taken when you introduce a mixture of compounds to be separated onto the absorbent column, Briefly describe any two. 3. Briefly compare any three advantages of column chromatography with those of thin-layer chromatography. 4. Briefly explain why TLC might not be or might be suitable for isolation of compounds that have boiling points below about 120C (at 760 torr)? 5. The Ai value of compound A is 0.36 when developed in petroleum ether and 0.47 when developed in ethyl acetate. Compound B has an P4 value of 0.42 in petroleum ether and 0.69 in chloroform. Which solvent would be better for separating a mixture of compounds A and B. Briefly explain your choice. 6. Carboxylic acids often streak on silica gel TLC plates. What might you add to the eluent to reduce streaking and why the streaking is reduced? 7. Rank in order of increasing eluting power for normal phase column chromatography ( 1= lowest eluting power; 5 = greatest eluting power) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


