Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please Help with this questions! QUESTION 13 A pickup truck has a fuel efficiency of 14.0 L/100 km. Assume complete combustion of a fuel that
Please Help with this questions! 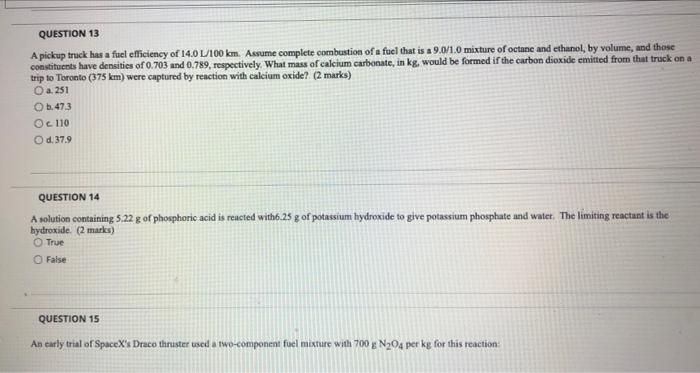
QUESTION 13 A pickup truck has a fuel efficiency of 14.0 L/100 km. Assume complete combustion of a fuel that is a 9.0/1.0 mixture of octane and ethanol, by volume, and those constituents have densities of 0.703 and 0.789, respectively. What mass of calcium carbonate, in kg, would be formed if the carbon dioxide emitted from that truck on a trip to Toronto (375 km) were captured by reaction with calcium oxide? (2 marks) O a 251 Ob.473 Oc 110 O d. 37.9 QUESTION 14 A solution containing 5.22 g of phosphoric acid is reacted with6.25 g of potassium hydroxide to give potassium phosphate and water. The limiting reactant is the bydroxide. (2 marks) True False QUESTION 15 An early trial of SpaceX's Draco thruster used a two-component fuel mixture with 700 g N204 per kg for this reaction 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


