Question
1. a.* Mg(s) + MgCl(aq) + AgNO3(aq) + _CaCl(aq) = _AgCl(s) + _CH(g) + _O(g) _CO(g) + _HO(g) _NaO(s) + _HO) _KCIO,(s) Al(s) +
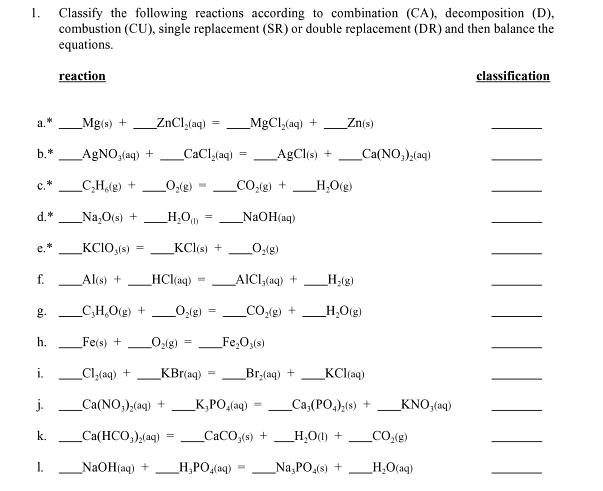
1. a.* Mg(s) + MgCl(aq) + AgNO3(aq) + _CaCl(aq) = _AgCl(s) + _CH(g) + _O(g) _CO(g) + _HO(g) _NaO(s) + _HO) _KCIO,(s) Al(s) + HCl(aq) g. CHO(g) + O(g) h. b.* C.* d.* e.* f. i. j. k. Classify the following reactions according to combination (CA), decomposition (D), combustion (CU), single replacement (SR) or double replacement (DR) and then balance the equations. reaction 1. _Fe(s) + _Cl(aq) + ZnCl(aq) KCl(s) + _O(g) = KBr(aq) _Ca(NO3)(aq) + = Ca(HCO3)(aq) = _NaOH(aq) + = NaOH(aq) _O(g) AICI,(aq) + _CO(g) + _FeO3(s) _HPO4(aq) Br(aq) + K,PO(aq) _CaCO (s) + Zn(s) Ca(NO)(aq) _H(g) _HO(g) KCl(aq) _Ca,(PO4)(s) + _HO(1) + _NaPO4(s) + KNO,(aq) CO(g) _H_O(aq) classification
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
a The given chemical reaction is as follows Mgs ZnCl 2 MgCl 2 aq Zns A balanced chemical reaction al...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



