Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please include what the option the answer is. thank you ! Multiple choice questions: 1. The molecules shown here: CH, C.H. and CzHs a) Are
please include what the option the answer is. thank you ! 

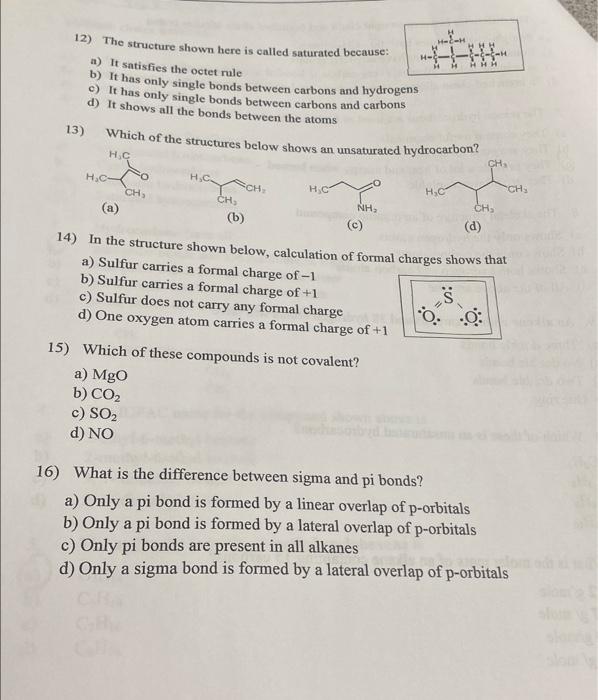
Multiple choice questions: 1. The molecules shown here: CH, C.H. and CzHs a) Are isomers of each other Are part of a homologous series Are all alkenes d) Have the general formula C2H2. 2. b) Another name for alkanes is Unsaturated hydrocarbons Cyclic hydrocarbons Aromatic hydrocarbons Saturated hydrocarbons Hydrocarbons have all: non-polar bonds and are insoluble in water non-polar bonds and are soluble in water polar bonds and are insoluble in water polar bonds and are soluble in water c) d) 4. CH, CH,CHCH,CH,CH,CHCH, CH, CH, The IUPAC name for the compound shown above is 2-ethyl-6-methyl heptane 2-methyl-6-ethyl heptane 3,7-dimethyl octane 2,6-dimethyl octane a) b) c) d) 5. a) The molecular formula of methyl cyclohexane is C H14 C6H14 C7H16 C3H16 d) Name: D 6. The difference between chain chain is that a) Straight-chain skave noch b) Benched-chain alkanes vero c) In branched- ches Ver todo che d) In branched-chaves, some are bedroom 7. The entrydrocarbure used as fuels is that *) They contain useful chemicals They are good for health They provide a lot of hest and energy when we d) They are all water soluble * The skeletal structure of an organic compound a) Shows only the hydrogen soms b) Shows only the carbon atoms -) Shows both hydrogen and carbon atoms d) All of the above are true 9. An alkene with 20 carbons and 38 hydrogen utoms will have a) Three double bonds b) No double bonds c) Two double bonds d) One ring 10) Which of these is an unsaturated hydrocarbon? a) CH b) CH CH d) C&H10 11) What is the molar mass of an alkane containing 15 carbons? a) 212 g/mole b) 257 g/mole c) 180 g/mole d) 200 g/mole HH H- H 12) The structure shown here is called saturated because a) It satisfies the octet rule b) It has only single bonds between carbons and hydrogens c) It has only single bonds between carbons and carbons d) It shows all the bonds between the atoms 13) Which of the structures below shows an unsaturated hydrocarbon? . HC- H. . CH, CH (b) CH HC CH (a) NH CH, 14) In the structure shown below, calculation of formal charges shows that a) Sulfur carries a formal charge of -1 b) Sulfur carries a formal charge of +1 s c) Sulfur does not carry any formal charge lo : d) One oxygen atom carries a formal charge of +1 15) Which of these compounds is not covalent? a) MgO b) CO2 c) SO2 d) NO 16) What is the difference between sigma and pi bonds? a) Only a pi bond is formed by a linear overlap of p-orbitals b) Only a pi bond is formed by a lateral overlap of p-orbitals c) Only pi bonds are present in all alkanes d) Only a sigma bond is formed by a lateral overlap of p-orbitals 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


