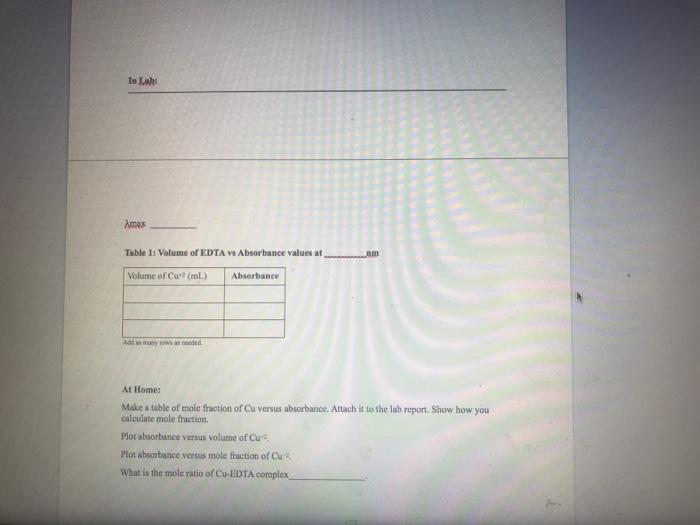
Please make calculations and draw graphs in excell,molarity of Cu+2&EDTA=5mM
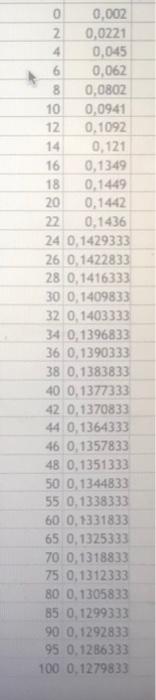
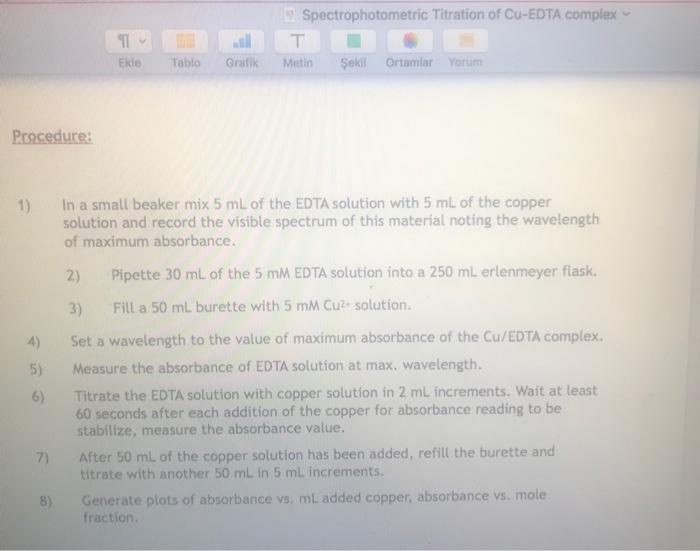
4.002 0.0221 - 0.045 0.062 0.0802 10 0.0941 0.92 12 16 0349 0449 20 0.14 12 0.436 24 01-2937, 26 0 1422837 28 0.1416333 300 140983, 201403333 34 0.1396633 36 0390333 38 0 1303633 40 0.137333 420,370833 40.1364333 46 0357837 46 0.135133 0 0 1144237 5 0.1338333 0 0.331333 650 152533 0 0.13188 0.1312333 180 0.132583 85 0.1299333 199 0.1292333 95 0.1285333 100 0.1279333 In : max Table 1: Volume of EDTA Vs Absorbance values at Volume of Cul (ml) Absorbance Added At Home: Make a table of mole fraction of Cu Versus absorbance. Attach it to the lab report. Show how you calculate mole fraction Plot absorbance sus vol of Cu Plot absorbance versus mole friction of Cu What is the mole ratio of Cu-EDTA complex Spectrophotometric Titration of Cu-EDTA complex T Metin Sokl Ortamlar Yorum Elde Tablo Gratik Procedure: 1) 4) In a small beaker mix 5 mL of the EDTA solution with 5 ml of the copper solution and record the visible spectrum of this material noting the wavelength of maximum absorbance. 2) Pipette 30 mL of the 5 mM EDTA solution into a 250 mL erlenmeyer flask. 3) Fill a 50 ml burette with 5 mm Cu2 solution Set a wavelength to the value of maximum absorbance of the Cu/EDTA complex, Measure the absorbance of EDTA solution at max. wavelength. Titrate the EDTA solution with copper solution in 2 mL increments. Wait at least 60 seconds after each addition of the copper for absorbance reading to be stabilize, measure the absorbance value. After 50 mL of the copper solution has been added, refill the burette and titrate with another 50 mL in 5 mL increments. Generate plots of absorbance vs. mL added copper, absorbance vs. mole fraction 5) 7) 8) 4.002 0.0221 - 0.045 0.062 0.0802 10 0.0941 0.92 12 16 0349 0449 20 0.14 12 0.436 24 01-2937, 26 0 1422837 28 0.1416333 300 140983, 201403333 34 0.1396633 36 0390333 38 0 1303633 40 0.137333 420,370833 40.1364333 46 0357837 46 0.135133 0 0 1144237 5 0.1338333 0 0.331333 650 152533 0 0.13188 0.1312333 180 0.132583 85 0.1299333 199 0.1292333 95 0.1285333 100 0.1279333 In : max Table 1: Volume of EDTA Vs Absorbance values at Volume of Cul (ml) Absorbance Added At Home: Make a table of mole fraction of Cu Versus absorbance. Attach it to the lab report. Show how you calculate mole fraction Plot absorbance sus vol of Cu Plot absorbance versus mole friction of Cu What is the mole ratio of Cu-EDTA complex Spectrophotometric Titration of Cu-EDTA complex T Metin Sokl Ortamlar Yorum Elde Tablo Gratik Procedure: 1) 4) In a small beaker mix 5 mL of the EDTA solution with 5 ml of the copper solution and record the visible spectrum of this material noting the wavelength of maximum absorbance. 2) Pipette 30 mL of the 5 mM EDTA solution into a 250 mL erlenmeyer flask. 3) Fill a 50 ml burette with 5 mm Cu2 solution Set a wavelength to the value of maximum absorbance of the Cu/EDTA complex, Measure the absorbance of EDTA solution at max. wavelength. Titrate the EDTA solution with copper solution in 2 mL increments. Wait at least 60 seconds after each addition of the copper for absorbance reading to be stabilize, measure the absorbance value. After 50 mL of the copper solution has been added, refill the burette and titrate with another 50 mL in 5 mL increments. Generate plots of absorbance vs. mL added copper, absorbance vs. mole fraction 5) 7) 8)