Answered step by step
Verified Expert Solution
Question
1 Approved Answer
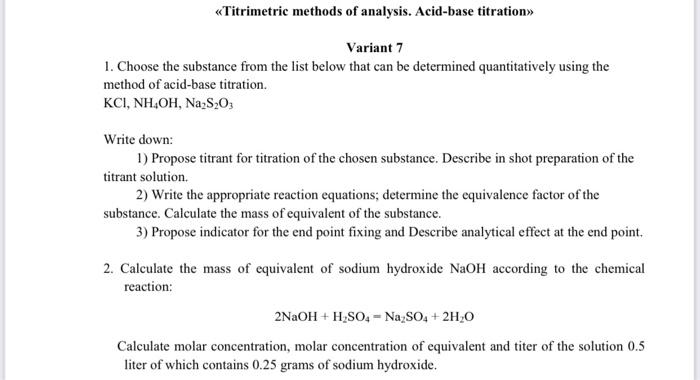
please need this urgently! for my exam! Titrimetric methods of analysis. Acid-base titration> Variant 7 1. Choose the substance from the list below that can
please need this urgently! for my exam!
Titrimetric methods of analysis. Acid-base titration> Variant 7 1. Choose the substance from the list below that can be determined quantitatively using the method of acid-base titration. KCI, NH4OH, Na2S2O Write down: 1) Propose titrant for titration of the chosen substance. Describe in shot preparation of the titrant solution. 2) Write the appropriate reaction equations; determine the equivalence factor of the substance Calculate the mass of equivalent of the substance. 3) Propose indicator for the end point fixing and Describe analytical effect at the end point 2. Calculate the mass of equivalent of sodium hydroxide NaOH according to the chemical reaction: 2NaOH + H2SO4-Na2SO4 + 2H20 Calculate molar concentration, molar concentration of equivalent and titer of the solution 0.5 liter of which contains 0.25 grams of sodium hydroxide Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


