Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please note the INCORRECT answers on the bottom Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular
please note the INCORRECT answers on the bottom 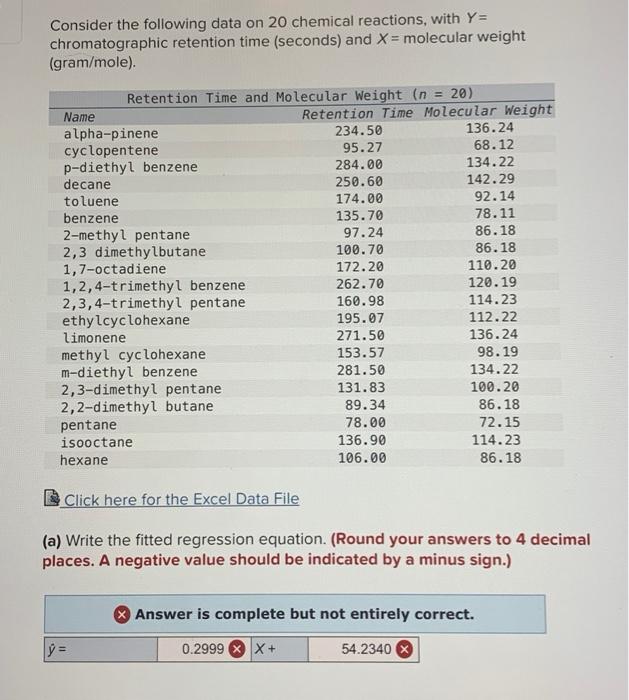
Consider the following data on 20 chemical reactions, with Y= chromatographic retention time (seconds) and X= molecular weight (gram/mole). Retention Time and Molecular Weight (n = 20) Name Retention Time Molecular Weight alpha-pinene 234.50 136.24 cyclopentene 95.27 68.12 p-diethyl benzene 284.00 134.22 decane 250.60 142.29 toluene 174.00 92.14 benzene 135.70 78.11 2-methyl pentane 97.24 86.18 2,3 dimethylbutane 100.70 86.18 1,7-octadiene 172.20 110.20 1,2,4-trimethyl benzene 262.70 120.19 2,3,4-trimethyl pentane 160.98 114.23 ethylcyclohexane 195.07 112.22 limonene 271.50 136.24 methyl cyclohexane 153.57 98.19 m-diethyl benzene 281.50 134.22 2,3-dimethyl pentane 131.83 100.20 2,2-dimethyl butane 89.34 86.18 pentane 78.00 72.15 isooctane 136.90 114.23 hexane 106.00 86.18 Click here for the Excel Data File (a) Write the fitted regression equation. (Round your answers to 4 decimal places. A negative value should be indicated by a minus sign.) Answer is complete but not entirely correct. 11 y 0.2999 X + 54.2340 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


