Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please please give me detailed answers of all parts i have assignmemt due in two hours uestion question 5 ( 1 0 marks ) The
please please give me detailed answers of all parts i have assignmemt due in two hours uestion question marks
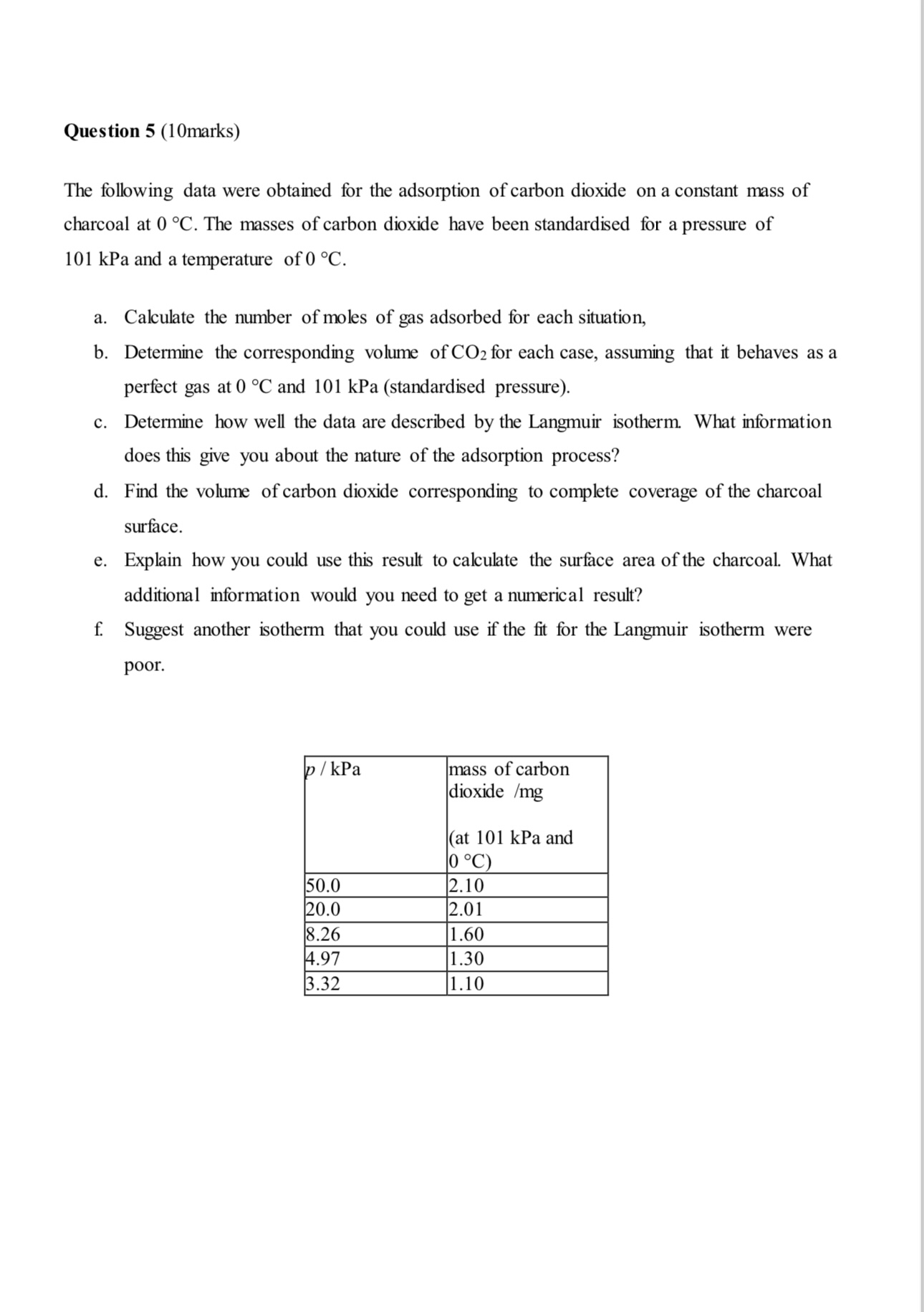
The following data were obtained for the adsorption of carbon dioxide on a constant mass of
charcoal at The masses of carbon dioxide have been standardised for a pressure of
kPa and a temperature of
a Calculate the number of moles of gas adsorbed for each situation,
b Determine the corresponding volume of for each case, assuming that it behaves as a
perfect gas at and kPa standardised pressure
c Determine how well the data are described by the Langmuir isotherm. What information
does this give you about the nature of the adsorption process?
d Find the volume of carbon dioxide corresponding to complete coverage of the charcoal
surface.
e Explain how you could use this result to calculate the surface area of the charcoal. What
additional information would you need to get a numerical result?
f Suggest another isotherm that you could use if the fit for the Langmuir isotherm were
poor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


