Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please please help Upload the spreadsheet file with the data and a plot for the cooling curves of solution of unknown fatty acid (second addition)
please please help 
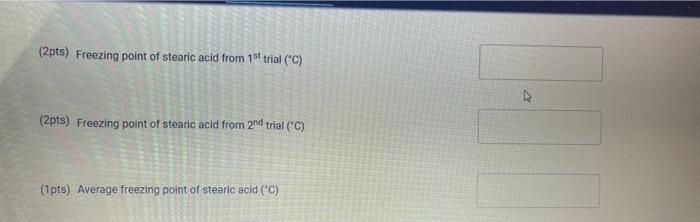
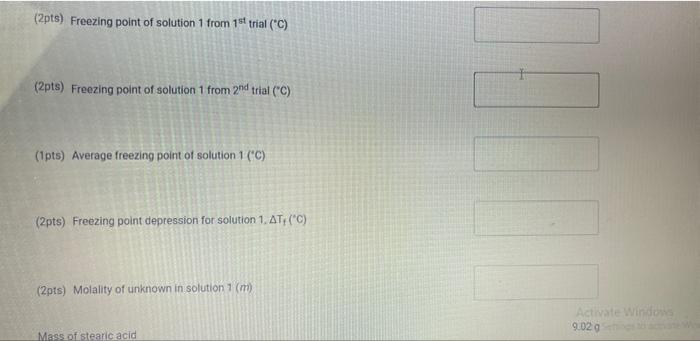

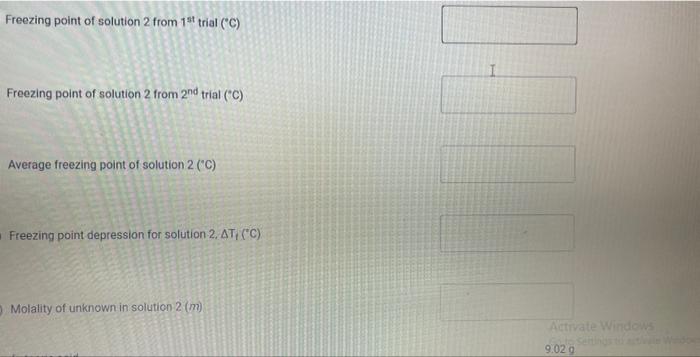


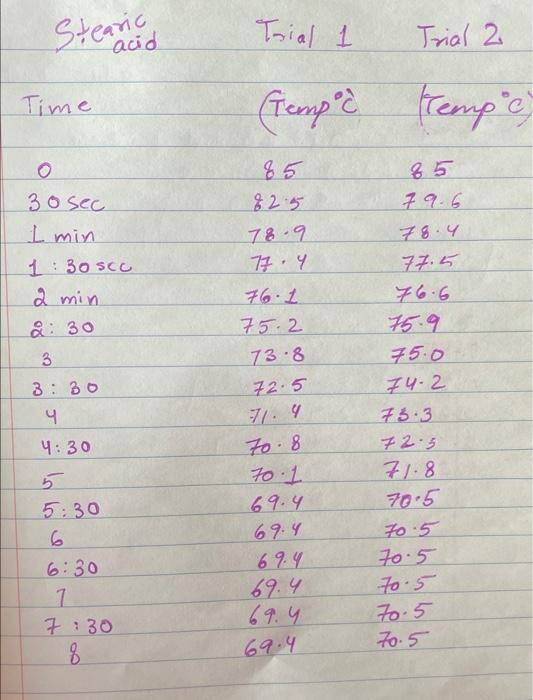
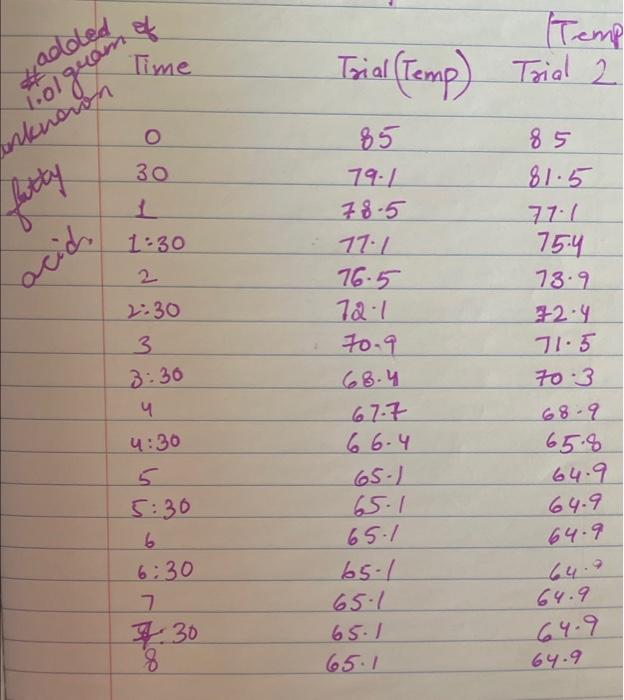
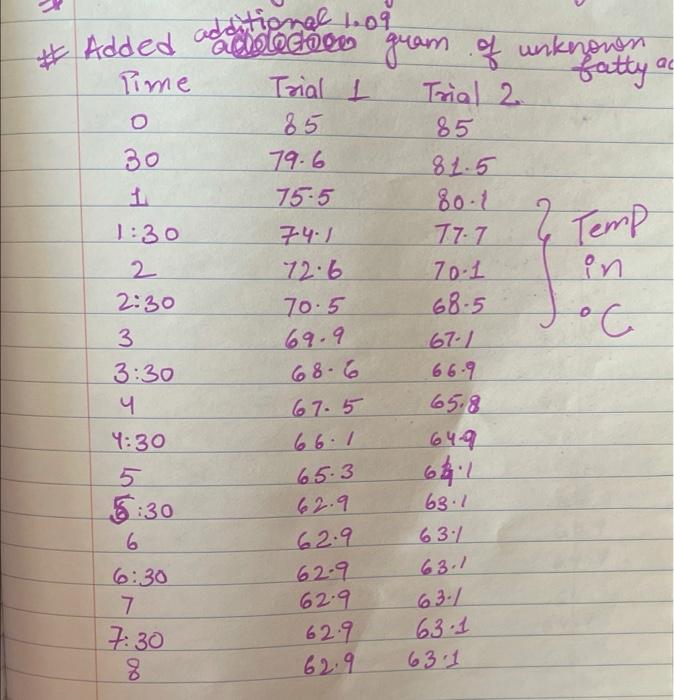
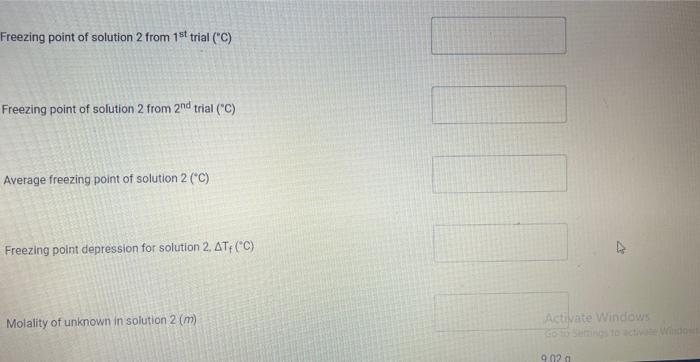
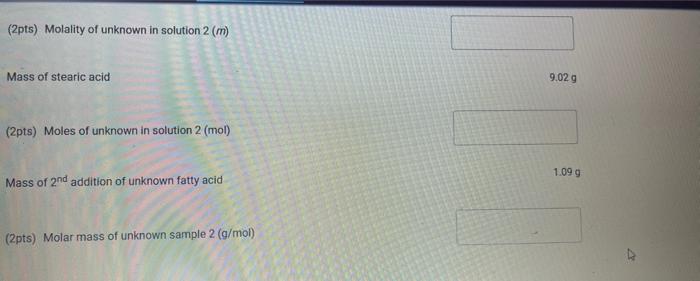
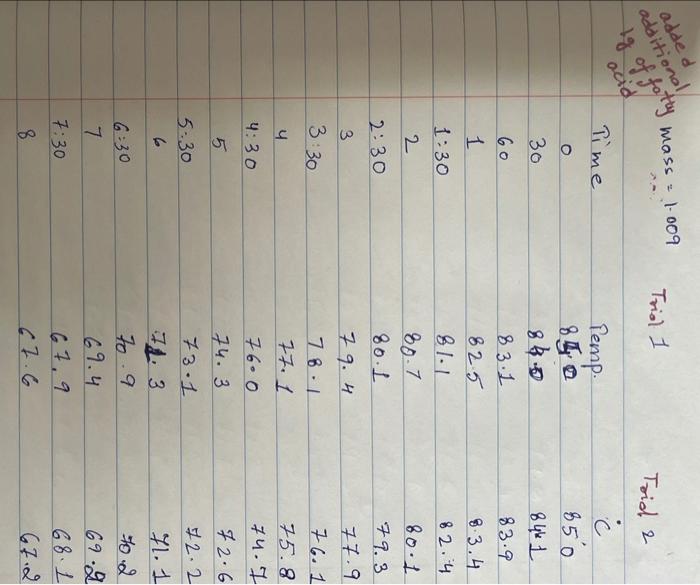
Upload the spreadsheet file with the data and a plot for the cooling curves of solution of unknown fatty acid (second addition) in stearic acid (2 trials on one graph) Browse your files to upload or Drag and drop Max attachments: 10 / Max Size 20.00MB each (5pts) Upload your calculations for freezing point of solution 2 for both trials Freezing point of solution 2 from 1st trial ("C) Freezing point of solution 2 from 2nd trial (C) Average freezing point of solution 2 (C) Freezing point depression for solution 2. AT: (C) Molality of unknown in solution 2 (m) Activate Windows 9020 (2pts) Molality of unknown in solution 2 (m) Mass of stearic acid 9.029 (2pts) Moles of unknown in solution 2 (mol) 1.099 Mass of 2nd addition of unknown fatty acid (2pts) Molar mass of unknown sample 2 (g/mol) (2pts) Average molar mass of unknown (g/mol) (3pts) What is the identity of your unknown? Normal BIU BB EIT LIV X X E = How does the additional amount of an unknown fatty acid sample change the freezing point of the solution? Briefly explain why? Normal BIU X, 1 X SIE falle EB FIT UIT T Compare your obtained molar mass with that of the expected molar mass of your unknown. What are the possible sources of error that may lead to the deviation from the desired molar mass? HIT Normal BIU EEE 2 XX Yule mass = 1-009 Trio 1 added additional) Yg of fatty acid Time O 30 60 1 1:30 2 2:30 3 Toid 2 850 846 1 83.9 8.3.4 82.4 80.1 79.3 77.9 76.1 75.8 74.7 72.6 72.2 71.1 to 2 69.9 68.1 67.2 Temp. 870 86.0 83.1 825 81.1 80.7 80.1 79.4 78.1 77.1 76.0 74.3 73.1 7. 3 7o.9 69.4 67.9 67.6 3.30 4 4:30 5 5:30 6 6:30 7 7:30 8 1. Mass of stearic acid (a) 9.02 Freezing Point of Solution 2. Code for Unknown fatty acid 1.01 3. Mass of 1st addition of unknown fatty acid (a) 4. Mass of 2nd addition of unknown fatty acid (a) 1.09 (20pts) Analyzing the Data Windo Freezing Point of the Solvent Upload the spreadsheet file with the data and a plot for the cooling curves of stearic acid (2 trials on one graph) Browse your files to upload or Drag and Drop Max attachments: 10 Max size: 20.00MB each (5pts) Upload your calculations of freezing point for both trials (2pts) Freezing point of stearic acid from 1st trial ("C) (2pts) Freezing point of stearic acid from 2nd trial (C) (1pts) Average freezing point of stearic acid (C) Upload the spreadsheet file with the data and a plot for the cooling curves of solution of unknown fatty acid (first addition) in stearic acid (2 trials on one graph) v Browse your files to upload or Drag and Drop Max attachments: 10 | Max Size: 20.00MB each (5pts) Upload your calculations for freezing point of solution 1 for both trials (2pts) Freezing point of solution 1 from 15 trial (C) TE (2pts) Freezing point of solution 1 from 2nd trial (C) (1pts) Average freezing point of solution 1 ("C) (2pts) Freezing point depression for solution 1. AT (C) (2pts) Molality of unknown in solution 1 (m) Activate Windows 9.029 Mass of stearic acid 9.029 Mass of stearic acid (2pts) Moles of unknown in solution 1 (mol) 1 1.019 Mass of 1st addition of unknown fatty acid (2pts) Molar mass of unknown sample 1 (g/mol) Upload the spreadsheet file with the data and a plot for the cooling curves of solution of unknown fatty acid (second addition) in stearic acid (2 trials on one graph) Browse your files to upload or Drag and Drop Max attachments. 10 | Max Size: 20.00MB each (5pts) Upload your calculations for freezing point of solution 2 for both trials Browse your files to upload Freezing point of solution 2 from 15 trial ("C) 1 Freezing point of solution 2 from 2nd trial ("C) Average freezing point of solution 2 (C) Freezing point depression for solution 2. AT (C) Molality of unknown in solution 2 (m) Activate Windows 9.029 (2pts) Moles of unknown in solution 2 (mol) Mass of 2nd addition of unknown fatty acid 1.099 (2pts) Molar mass of unknown sample 2 (g/mol) (2pts) Average molar mass of unknown (g/mol) (3pts) What is the identity of your unknown? How does the additional amount of an unknown fatty acid sample change the freezing point of the solution? Briefly explain why? Saved Normal BIU X, X ESE x IT ET 7 (2pts) Compare your obtained molar mass with that of the expected molar mass of your unknown. What are the possible sources of error that may lead to the deviation from the desired molar mass? Stearaad Trial 1 Trial 2 Time (Temp & tempoc 30 sec I min 1:30sco 2 min &: 30 85 82.5 78.9 77.4 76.1 75.2 73.8 72.5 3 3:30 S... 65 79.6 78.4 77.5 76.6 75.9 75.0 74.2 75.3 72.5 71.8 70.5 70.5 70.5 70.5 70.5 70.5 71.4 4:30 5:30 6 6:30 7 7:30 8 70.8 70.1 69.4 69.4 69.4 69.4 69.4 69.4 $ Temp Time #adoled 1. olguam Trial (Temp) Trial 2 2 anknown 30 fatty acid 1:30 2 2:30 3 3:30 4 85 79.1 78.5 77.1 76.5 72.1 70-9 68.4 67.7 6 6.4 65-1 65.1 65.1 65.1 65.1 65.1 65.1 85 81.5 77.1 75.4 73.9 72.4 71.5 70.3 68-9 65.8 64.9 64.9 4:30 64.9 5:30 6 6:30 7 7.30 8 64.9 64.9 64.9 additional 1:09 # Added Time fatty ac o Temp 30 1 1:30 2 2:30 3 3:30 4:30 Trial 1 85 79.6 75.5 74.1 72.6 70.5 69.9 68.6 67.5 66.1 65.3 62.9 62.9 62.9 62.9 62.9 62.9 gram of unknown Trial 2 85 81.5 80.1 2 77.7 70.1 in 68.5 C 67.1 66-9 65.8 649 68.1 63.1 63.1 5:30 6. 6:30 7 7:30 63.1 63.1 63.1 63.1 00 













Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


