Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please provide answers for all the parts of the question (a , b , c and d) Solve as fast as possible please 4. To
Please provide answers for all the parts of the question (a , b , c and d)
4. To titrate 25.00 mL of a solution of hydrogen peroxide (H2O2), in acidic buffer medium (pH= 1.00), a volume of 19.38 mL of a standard potassium permanganate solution was used 0.05000 mol/L. a) Write the half-equations and the overall chemical equation that translates the titration reaction. b) Calculate the concentration of hydrogen peroxide in the analyzed solution. c) Sketch the titration curve, indicating what is represented on each axis. d) Calculate the potential of an inert electrode in the titrant, after the addition of 25.00 mL of standard solution of KMnO4. Data: E (O2(g)/H2O2(aq)) = 0,68 V; E (MnO4 - (aq)/Mn2+(aq)) = 1,51 V Solve as fast as possible please 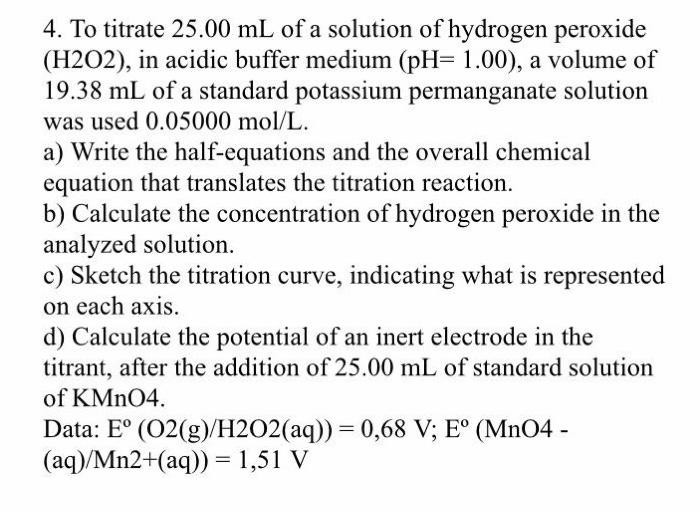

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


