Question
The internal energy U(T,P) and the specific Gibbs free energy G defined by: G = U + PV TS are state functions and therefore their
The internal energy U(T,P) and the specific Gibbs free energy G defined by: G = U + PV TS are state functions and therefore their expressions in the differential form, dU and dG, respectively, are differential Exact Sciences. From the differential form of the energy conservation equation for a closed system, containing a compressible fluid, the expression:
dU = TdS - PdV is obtained.
Using one of Maxwell's relations, the change in internal energy with respect to pressure, keeping the temperature constant,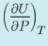 is determined by the expression:
is determined by the expression:
Choose an option:
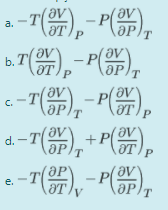
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


