Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE PROVIDE COMPREHENSIVE FULL ANSWER TO ALL PARTS. A+Bk2k12C The following reversible gas phase reaction follows an elementary rate law and is carriec out isothermally
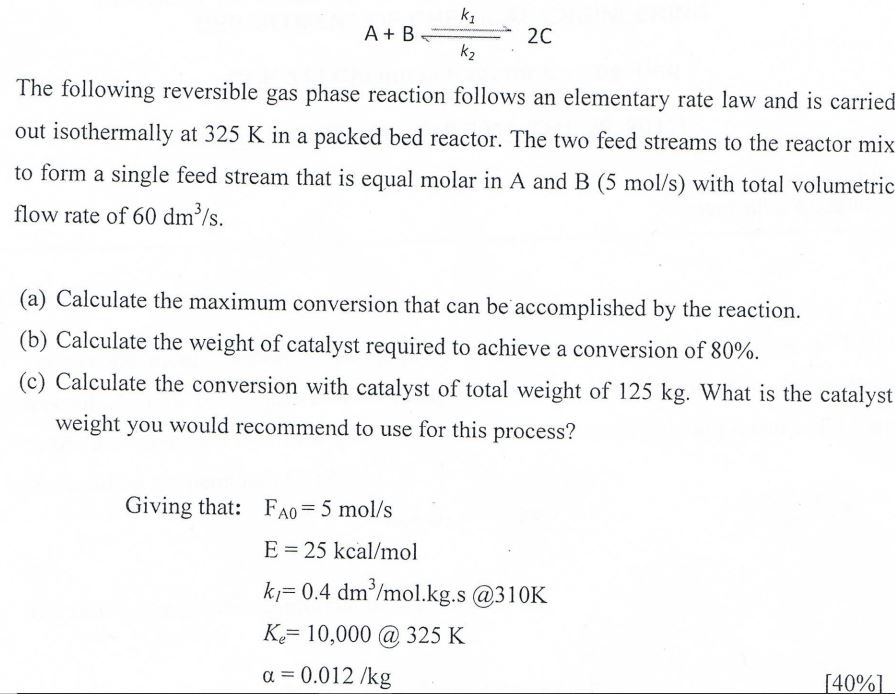
PLEASE PROVIDE COMPREHENSIVE FULL ANSWER TO ALL PARTS.
A+Bk2k12C The following reversible gas phase reaction follows an elementary rate law and is carriec out isothermally at 325K in a packed bed reactor. The two feed streams to the reactor mix to form a single feed stream that is equal molar in A and B(5mol/s) with total volumetric flow rate of 60dm3/s (a) Calculate the maximum conversion that can be accomplished by the reaction. (b) Calculate the weight of catalyst required to achieve a conversion of 80%. (c) Calculate the conversion with catalyst of total weight of 125kg. What is the catalyst weight you would recommend to use for this process? Givingthat:FA0=5mol/sE=25kcal/molkl=0.4dm3/mol.kg.s@310KKe=10,000@325K=0.012/kg [40%] A+Bk2k12C The following reversible gas phase reaction follows an elementary rate law and is carriec out isothermally at 325K in a packed bed reactor. The two feed streams to the reactor mix to form a single feed stream that is equal molar in A and B(5mol/s) with total volumetric flow rate of 60dm3/s (a) Calculate the maximum conversion that can be accomplished by the reaction. (b) Calculate the weight of catalyst required to achieve a conversion of 80%. (c) Calculate the conversion with catalyst of total weight of 125kg. What is the catalyst weight you would recommend to use for this process? Givingthat:FA0=5mol/sE=25kcal/molkl=0.4dm3/mol.kg.s@310KKe=10,000@325K=0.012/kg [40%]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


