Answered step by step
Verified Expert Solution
Question
1 Approved Answer
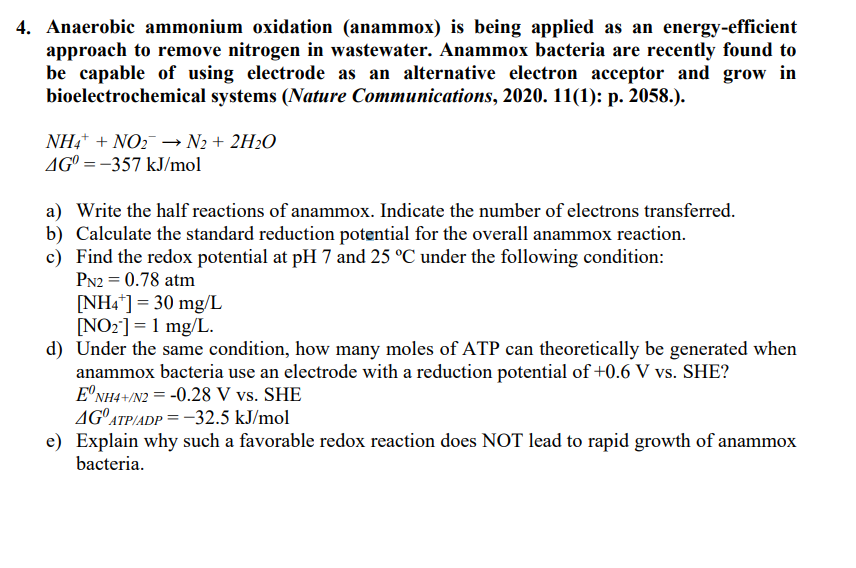
please provide detailed answe Anaerobic ammonium oxidation ( anammox ) is being applied as energy - efficient approach to remove nitrogen in wastewater. Anammox bacteria
please provide detailed answe Anaerobic ammonium oxidation anammox is being applied as energyefficient
approach to remove nitrogen in wastewater. Anammox bacteria are recently found to
be capable of using electrode as an alternative electron acceptor and grow in
bioelectrochemical systems Nature Communications, : p
a Write the half reactions of anammox. Indicate the number of electrons transferred.
b Calculate the standard reduction potential for the overall anammox reaction.
c Find the redox potential at and under the following condition:
atm
d Under the same condition, how many moles of ATP can theoretically be generated when
anammox bacteria use an electrode with a reduction potential of SHE?
SHE
e Explain why such a favorable redox reaction does NOT lead to rapid growth of anammox
bacteria.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


