Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please provide detailed answer. will upvote! Most household furnaces burn methane to produce heat. A mixture of hydrogen in methane is proposed to reduce greenhouse
please provide detailed answer. will upvote! 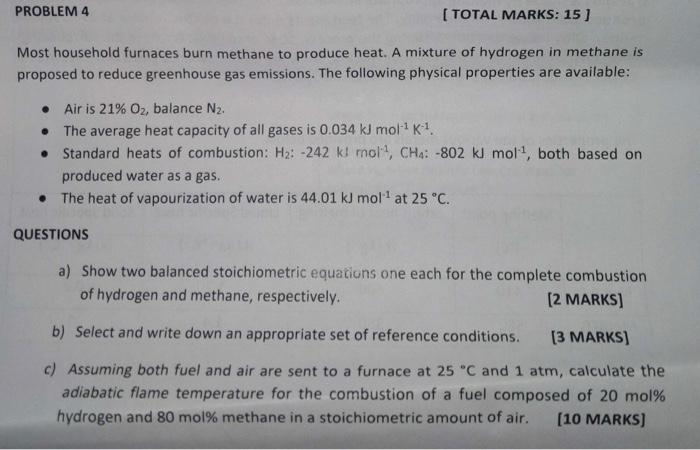
Most household furnaces burn methane to produce heat. A mixture of hydrogen in methane is proposed to reduce greenhouse gas emissions. The following physical properties are available: - Air is 21%O2, balance N2. - The average heat capacity of all gases is 0.034kJmol1K1. - Standard heats of combustion: H2:242kJmol1,CH4:802kJmol1, both based on produced water as a gas. - The heat of vapourization of water is 44.01kJmol1 at 25C. QUESTIONS a) Show two balanced stoichiometric equations one each for the complete combustion of hydrogen and methane, respectively. [2 MARKS] b) Select and write down an appropriate set of reference conditions. [3 MARKS] c) Assuming both fuel and air are sent to a furnace at 25C and 1atm, calculate the adiabatic flame temperature for the combustion of a fuel composed of 20 mol\% hydrogen and 80 mol\% methane in a stoichiometric amount of air. [10 MARKS] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


