Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please provide step by step of how to graph 5-44 Use the diffusion data in the table below for atoms in iron to answer the
Please provide step by step of how to graph
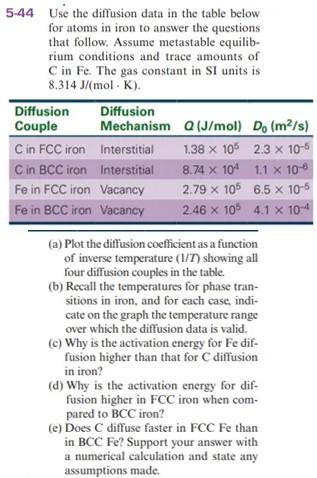
5-44 Use the diffusion data in the table below for atoms in iron to answer the questions that follow. Assume metastable equilib- rium conditions and trace amounts of C in Fe. The gas constant in SI units is 8.314 J/mol K). Diffusion Diffusion Couple Mechanism (J/mol) D (m/s) Q , s) Cin FCC iron Interstitial 1.38 x 106 2.3 x 10-6 Cin BCC iron Interstitial 8.74 X 104 1.1 x 10-6 Fein FCC iron Vacancy 2.79 X 105 6.5 X 10-5 Fe in BCC iron Vacancy 2.46 x 106 4.1 x 10-4 (a) Plot the diffusion coefficient as a function of inverse temperature (1/7) showing all four diffusion couples in the table. (b) Recall the temperatures for phase tran- sitions in iron, and for each case, indi- cate on the graph the temperature range over which the diffusion data is valid. (c) Why is the activation energy for Fe dif- fusion higher than that for C diffusion in iron? (d) Why is the activation energy for dif- fusion higher in FCC iron when com- pared to BCC iron? (e) Does C diffuse faster in FCC Fe than in BCC Fe? Support your answer with a numerical calculation and state any assumptions madeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


