Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please provide steps by step solution so that I can follow thank you 2. Consider the following reaction mechanism. S+AK1A2dAadK4K3BadBadK5BB+S Where the Ks (K1K6) represent
please provide steps by step solution so that I can follow thank you
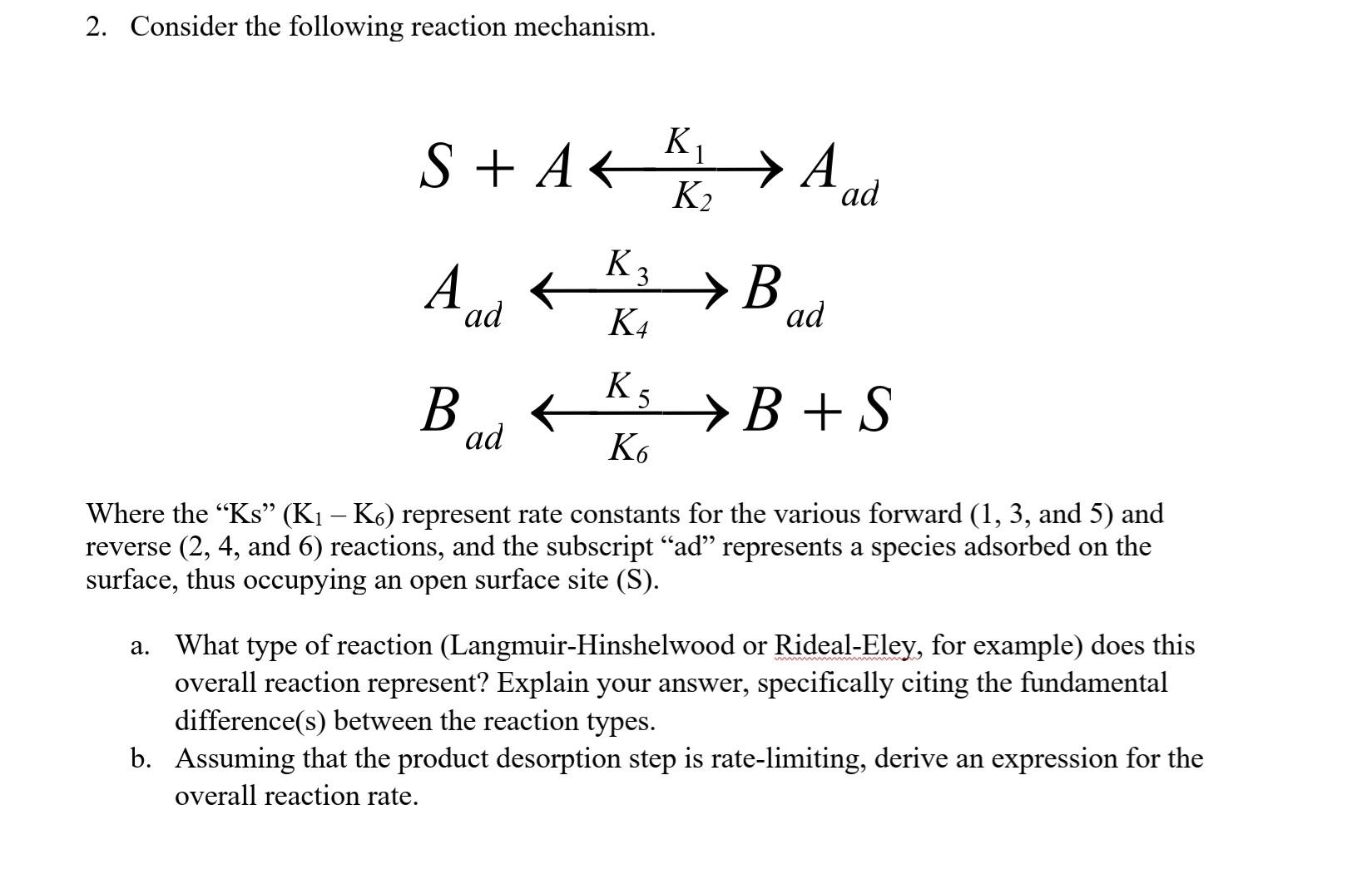
2. Consider the following reaction mechanism. S+AK1A2dAadK4K3BadBadK5BB+S Where the "Ks" (K1K6) represent rate constants for the various forward (1, 3, and 5) and reverse (2,4, and 6) reactions, and the subscript "ad" represents a species adsorbed on the surface, thus occupying an open surface site (S). a. What type of reaction (Langmuir-Hinshelwood or Rideal-Eley, for example) does this overall reaction represent? Explain your answer, specifically citing the fundamental difference(s) between the reaction types. b. Assuming that the product desorption step is rate-limiting, derive an expression for the overall reaction rate
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


