Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please respond to both ill give a good review The second-order reaction 2 Mn(CO) -- Mn,Cho has a rate constant equal to 3.0 X 10'M's
please respond to both ill give a good review 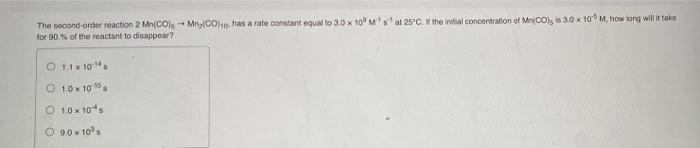
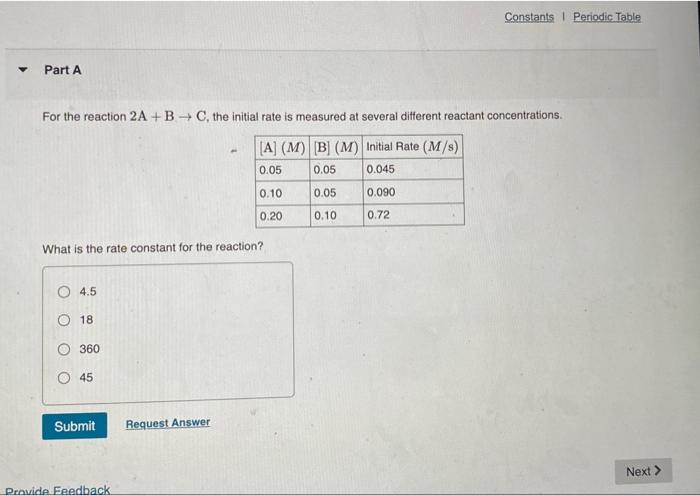
The second-order reaction 2 Mn(CO) -- Mn,Cho has a rate constant equal to 3.0 X 10'M's at 25C, the initial concertiration of Mnico) 83.0 x 10M, how long will it tako for 90.5 of the reactant to disappear? O 1.1x10. O 10 X 108 1,0 10-15 O 9.0x10 Constants Periodic Table Part A For the reaction 2A + BC, the initial rate is measured at several different reactant concentrations. [A] (M) [B] (M) Initial Rate (M/s) 0.05 0.05 0.045 0.10 0.05 0.090 0.20 0.10 0.72 What is the rate constant for the reaction? O 4.5 O 18 360 45 Submit Request Answer Next > Provide Feedback 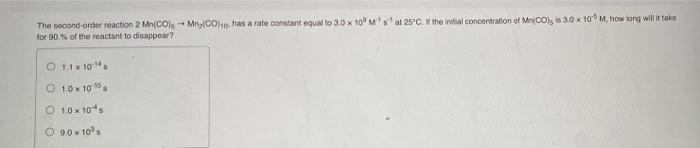

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


