Question: Please show all steps and solve legibly if not in typed form. Thank you 0.200g sample of high purity Cu(s) was dissolved in concentrated nitric
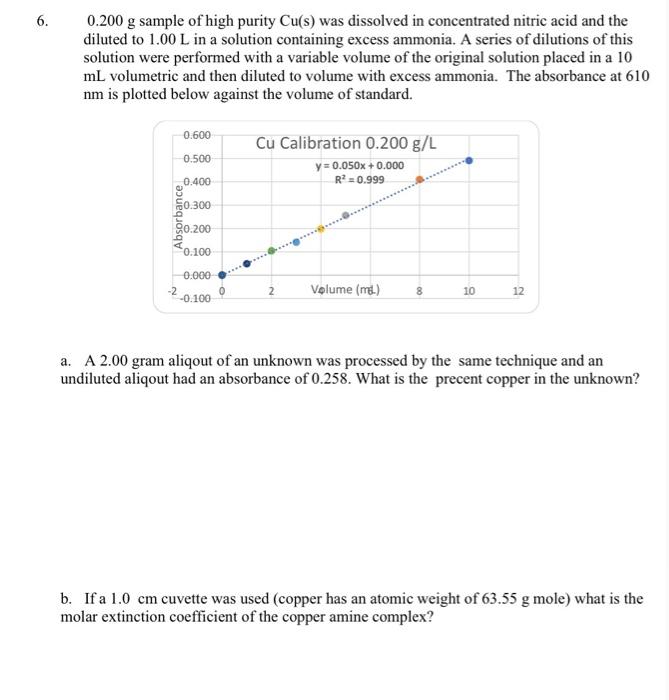
0.200g sample of high purity Cu(s) was dissolved in concentrated nitric acid and the diluted to 1.00L in a solution containing excess ammonia. A series of dilutions of this solution were performed with a variable volume of the original solution placed in a 10 mL volumetric and then diluted to volume with excess ammonia. The absorbance at 610 nm is plotted below against the volume of standard. a. A 2.00 gram aliqout of an unknown was processed by the same technique and an undiluted aliqout had an absorbance of 0.258. What is the precent copper in the unknown? b. If a 1.0cm cuvette was used (copper has an atomic weight of 63.55g mole) what is the molar extinction coefficient of the copper amine complex
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


