Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show all the steps Thank you! ! Required information The values of the ideal gas constant (R) are given in the following table: Values
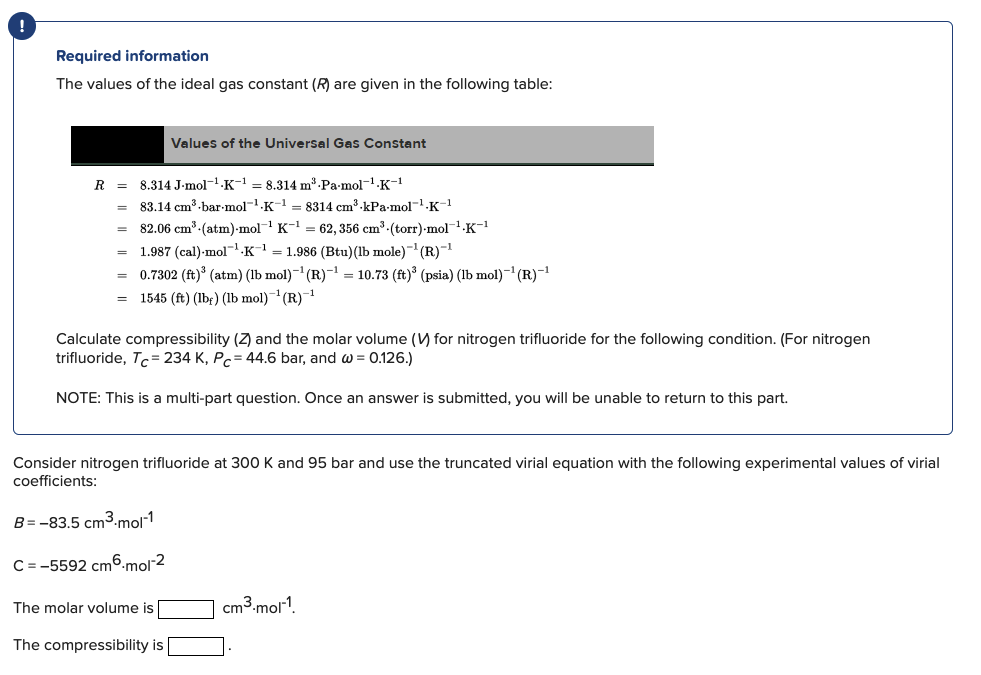


Please show all the steps Thank you!
! Required information The values of the ideal gas constant (R) are given in the following table: Values of the Universal Gas Constant R = 8.314 J-mol-1-K-1 = 8.314 m Pa.mol-1-K-1 83.14 cm-bar.mol-1.K-1 = 8314 cm kPa-mol-.K-1 82.06 cm (atm)-mol K-1 = 62, 356 cm (torr) mol K-1 1.987 (cal).mol-?K = 1.986 (Btu)(Ib mole)- (R)-1 = 0.7302 (ft)' (atm) (lb mol)-(R)-= 10.73 (ft) (psia) (lb mol)-(R)-1 1545 (ft) (lbf) (lb mol)--(R)-1 = Calculate compressibility (7 and the molar volume (V) for nitrogen trifluoride for the following condition. (For nitrogen trifluoride, Tc= 234 K, Pc = 44.6 bar, and w = 0.126.) NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. 300 and 95 bar use the trur Consider nitrogen trifluoride coefficients: virial equation following experimental B = -83.5 cm3.mol-1 C = -5592 cm 6-mol-2 The molar volume is cm3.mol-? The compressibility is Consider nitrogen trifluoride at 300 K and 92 bar and use the truncated virial equation with a value of B from the generalized Pitzer correlation. The molar volume is cm3.mol-? The compressibility is Consider nitrogen trifluoride at 300 K and 95 bar and use the Redlich/Kwong equation. The molar volume is cm3.mol? The compressibility isStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


