Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show all work and answer all parts 5. (32%) An industrially important enzyme (E) catalyzes the conversion of a protein substrate (S) to a
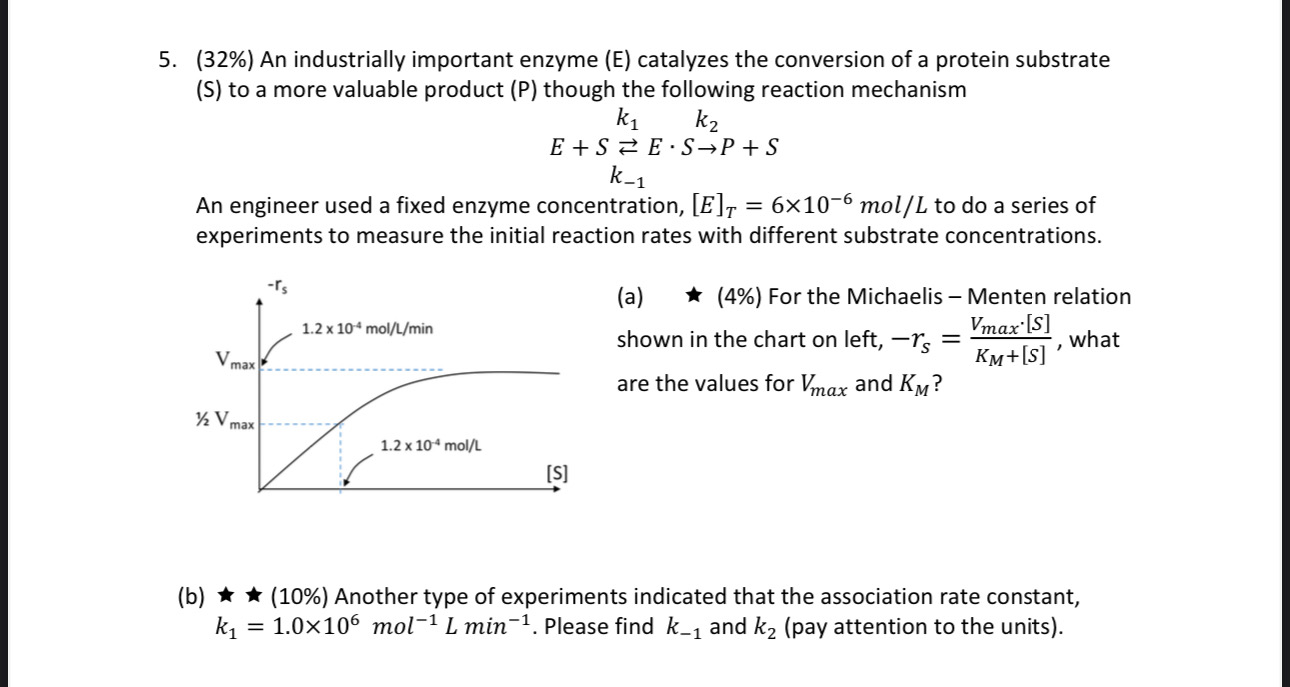
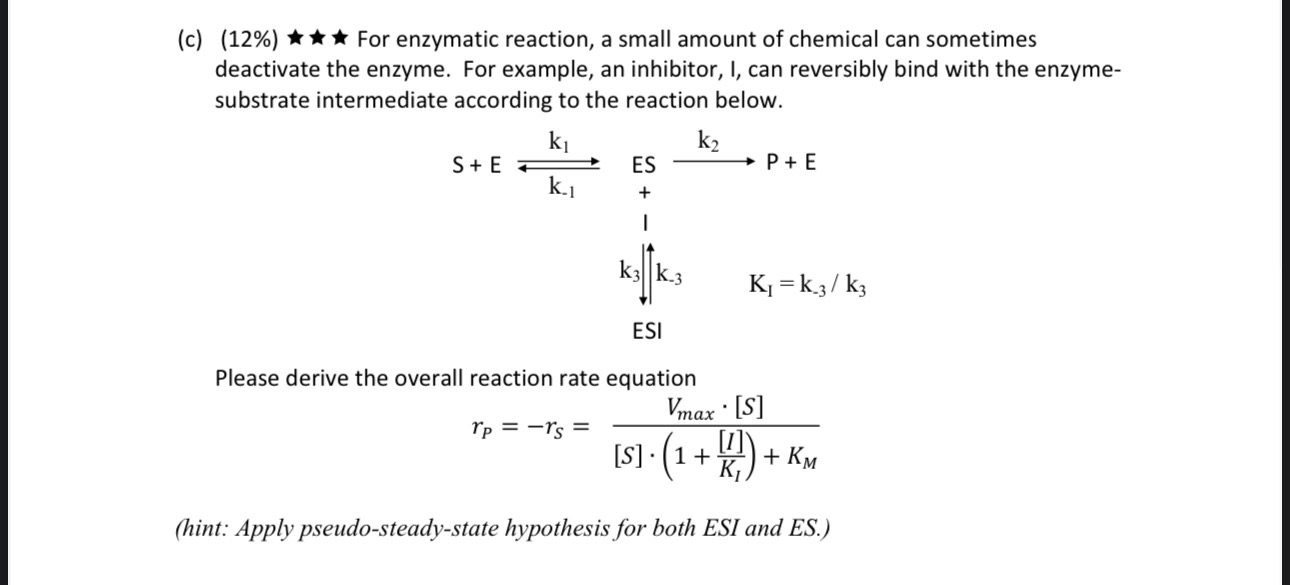
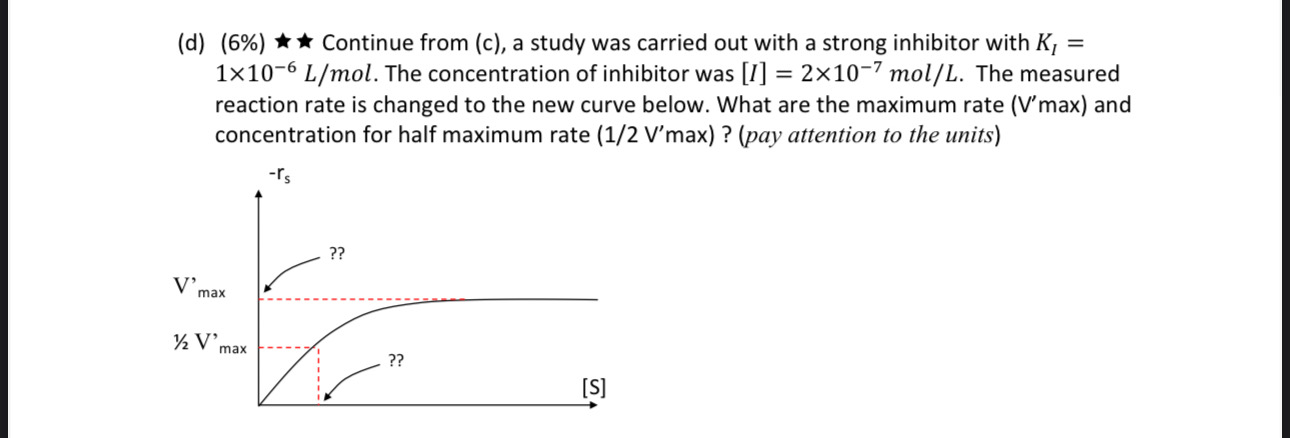
Please show all work and answer all parts
5. (32\%) An industrially important enzyme (E) catalyzes the conversion of a protein substrate (S) to a more valuable product (P) though the following reaction mechanism E+Sk1k1ESP+S An engineer used a fixed enzyme concentration, [E]T=6106mol/L to do a series of experiments to measure the initial reaction rates with different substrate concentrations. (a) (4\%) For the Michaelis - Menten relation shown in the chart on left, rS=KM+[S]Vmax[S], what are the values for Vmax and KM ? (b) (10\%) Another type of experiments indicated that the association rate constant, k1=1.0106mol1Lmin1. Please find k1 and k2 (pay attention to the units). (c) (12%) For enzymatic reaction, a small amount of chemical can sometimes deactivate the enzyme. For example, an inhibitor, I, can reversibly bind with the enzymesubstrate intermediate according to the reaction below. k1S+kESk2+P+Ek3]k3ESIKI=k3/k3 Please derive the overall reaction rate equation rP=rS=[S](1+KI[I])+KMVmax[S] (hint: Apply pseudo-steady-state hypothesis for both ESI and ES.) (d) (6%) Continue from (c), a study was carried out with a strong inhibitor with KI= 1106L/mol. The concentration of inhibitor was [I]=2107mol/L. The measured reaction rate is changed to the new curve below. What are the maximum rate ( V max) and concentration for half maximum rate ( 1/2Vmax) ? (pay attention to the units) 5. (32\%) An industrially important enzyme (E) catalyzes the conversion of a protein substrate (S) to a more valuable product (P) though the following reaction mechanism E+Sk1k1ESP+S An engineer used a fixed enzyme concentration, [E]T=6106mol/L to do a series of experiments to measure the initial reaction rates with different substrate concentrations. (a) (4\%) For the Michaelis - Menten relation shown in the chart on left, rS=KM+[S]Vmax[S], what are the values for Vmax and KM ? (b) (10\%) Another type of experiments indicated that the association rate constant, k1=1.0106mol1Lmin1. Please find k1 and k2 (pay attention to the units). (c) (12%) For enzymatic reaction, a small amount of chemical can sometimes deactivate the enzyme. For example, an inhibitor, I, can reversibly bind with the enzymesubstrate intermediate according to the reaction below. k1S+kESk2+P+Ek3]k3ESIKI=k3/k3 Please derive the overall reaction rate equation rP=rS=[S](1+KI[I])+KMVmax[S] (hint: Apply pseudo-steady-state hypothesis for both ESI and ES.) (d) (6%) Continue from (c), a study was carried out with a strong inhibitor with KI= 1106L/mol. The concentration of inhibitor was [I]=2107mol/L. The measured reaction rate is changed to the new curve below. What are the maximum rate ( V max) and concentration for half maximum rate ( 1/2Vmax) ? (pay attention to the units)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


