Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show calculation work please 9. The mass flow rate of the first gaseous feed stream, containing the component I only, is 6,000g/s. The total
please show calculation work please 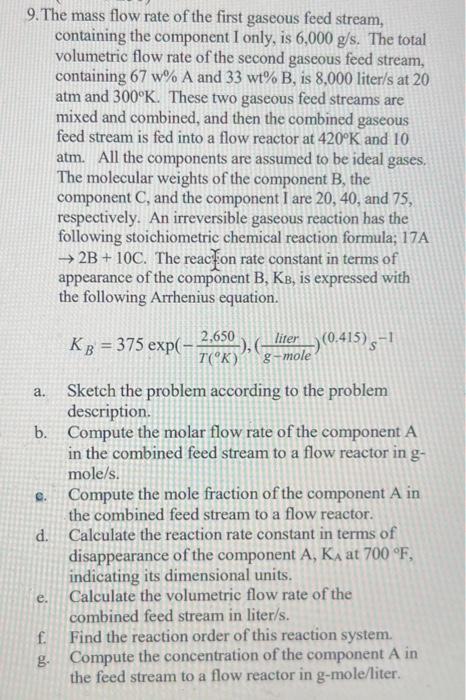
9. The mass flow rate of the first gaseous feed stream, containing the component I only, is 6,000g/s. The total volumetric flow rate of the second gaseous feed stream, containing 67w%A and 33wt%B, is 8,000 liter/s at 20 atm and 300K. These two gaseous feed streams are mixed and combined, and then the combined gaseous feed stream is fed into a flow reactor at 420K and 10 atm. All the components are assumed to be ideal gases. The molecular weights of the component B, the component C, and the component I are 20,40 , and 75 , respectively. An irreversible gaseous reaction has the following stoichiometric chemical reaction formula; 17A 2B+10C. The reacy. appearance of the component B,KB, is expressed with the following Arrhenius equation. KB=375exp(T(K)2,650),(gmoleliter)(0.415)s1 a. Sketch the problem according to the problem description. b. Compute the molar flow rate of the component A in the combined feed stream to a flow reactor in g mole/s. c. Compute the mole fraction of the component A in the combined feed stream to a flow reactor. d. Calculate the reaction rate constant in terms of disappearance of the component A,KA at 700F, indicating its dimensional units. e. Calculate the volumetric flow rate of the combined feed stream in liter/s. f. Find the reaction order of this reaction system. g. Compute the concentration of the component A in the feed stream to a flow reactor in g-mole/liter 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


