Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show how to do number 8, 9, 10 A dose of Brand Y cough syrup has a mass of 28.0g and contains 0.550g acetaminophen.
please show how to do number 8, 9, 10 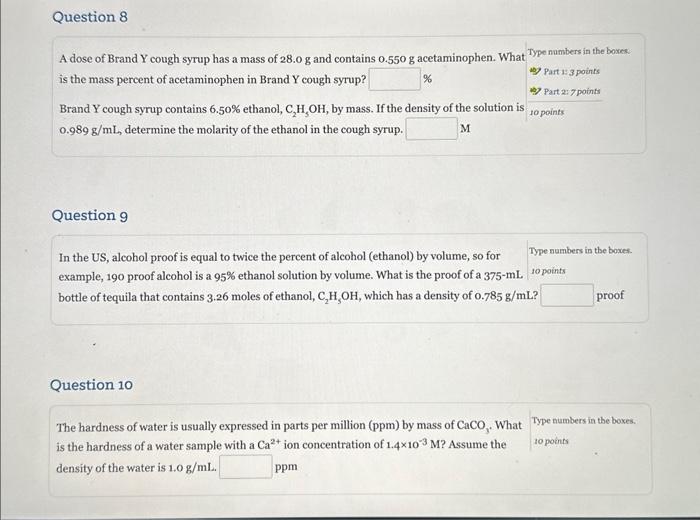
A dose of Brand Y cough syrup has a mass of 28.0g and contains 0.550g acetaminophen. What Type nambers in the boxes: is the mass percent of acetaminophen in Brand Y cough syrup? 48 Part 13 points Brand Y cough syrup contains 6.50% ethanol, C2H3OH, by mass. If the density of the solution is points 0.989g/mL, determine the molarity of the ethanol in the cough syrup. M Question 9 In the US, alcohol proof is equal to twice the percent of alcohol (ethanol) by volume, so for example, 190 proof alcohol is a 95% ethanol solution by volume. What is the proof of a 375mL topoints bottle of tequila that contains 3.26 moles of ethanol, C2H5OH, which has a density of 0.785g/mL ? proof Question 10 The hardness of water is usually expressed in parts per million (ppm) by mass of CaCO3. What Type numbers in the boxes. is the hardness of a water sample with a Ca2+ ion concentration of 1.4103M ? Assume the density of the water is 1.0g/mL. ppm 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


