Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show how to find mass and moles expected for this reaction - Reduction of Fluorenone mass moles mass expected expected usea moles! used density
please show how to find mass and moles expected for this reaction - Reduction of Fluorenone 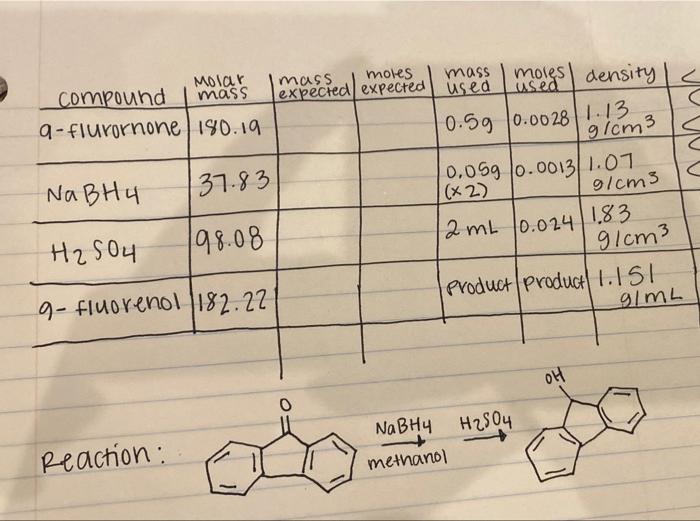
mass moles mass expected expected usea moles! used density C C Molar compound mass a-flurornone / 180.19 0.59 0.0028 1.13 g/cm3 Na BH u 37.83 H2SO4 198.08 0.059 0.00131.01 (x2) g/cm3 12 mL 10.024 1.83 g/cm3 Product Product) 1.151 g/mL q- fluorenol 1182.22 OH NaBH4 H2SO4 methanol Reaction 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


