Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show work To the stratosphere To tho stratosphere 50 HCHO 17 0 OH andation 240 00 1200 g protokon OH Caddebon CO2 1750.000 TCW
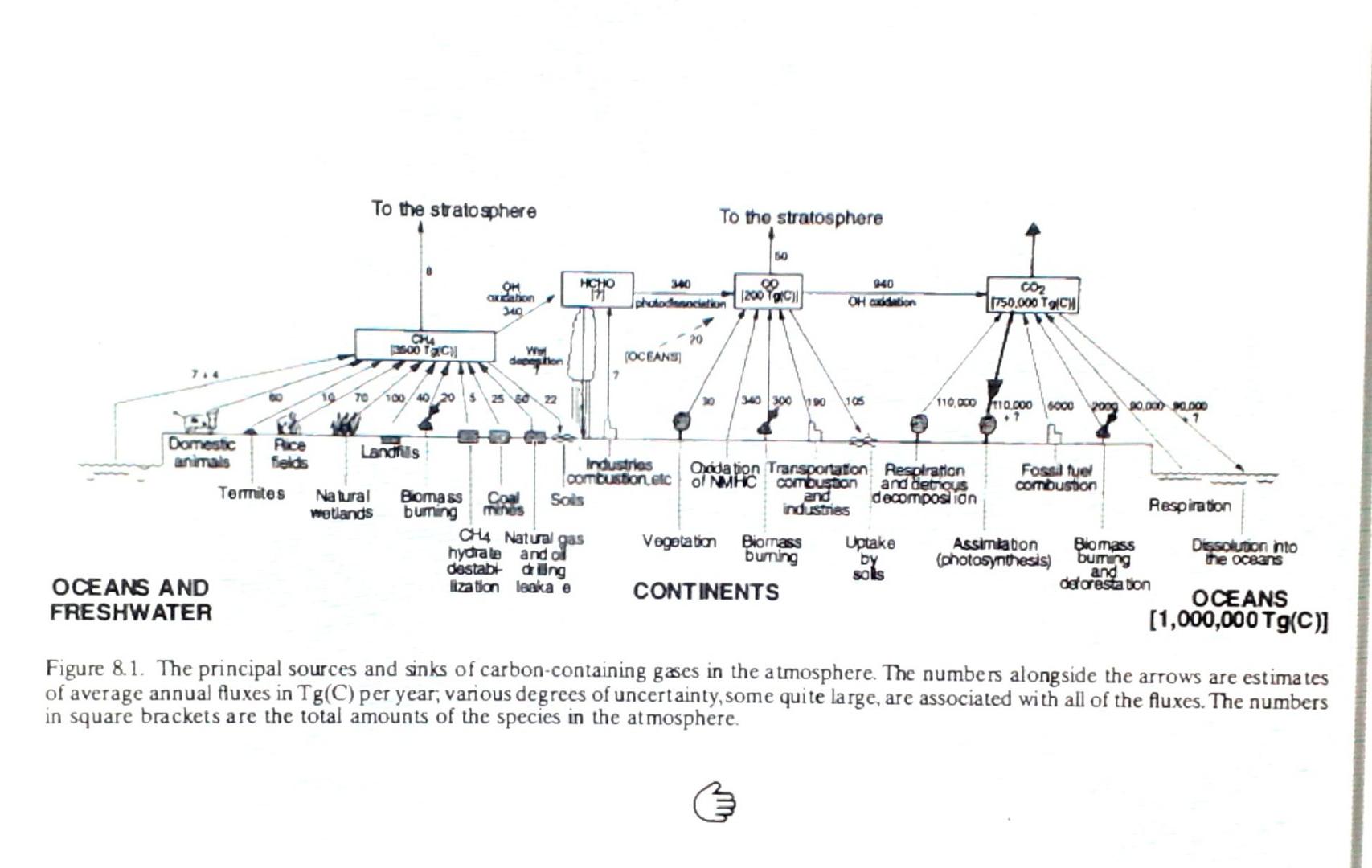
please show work
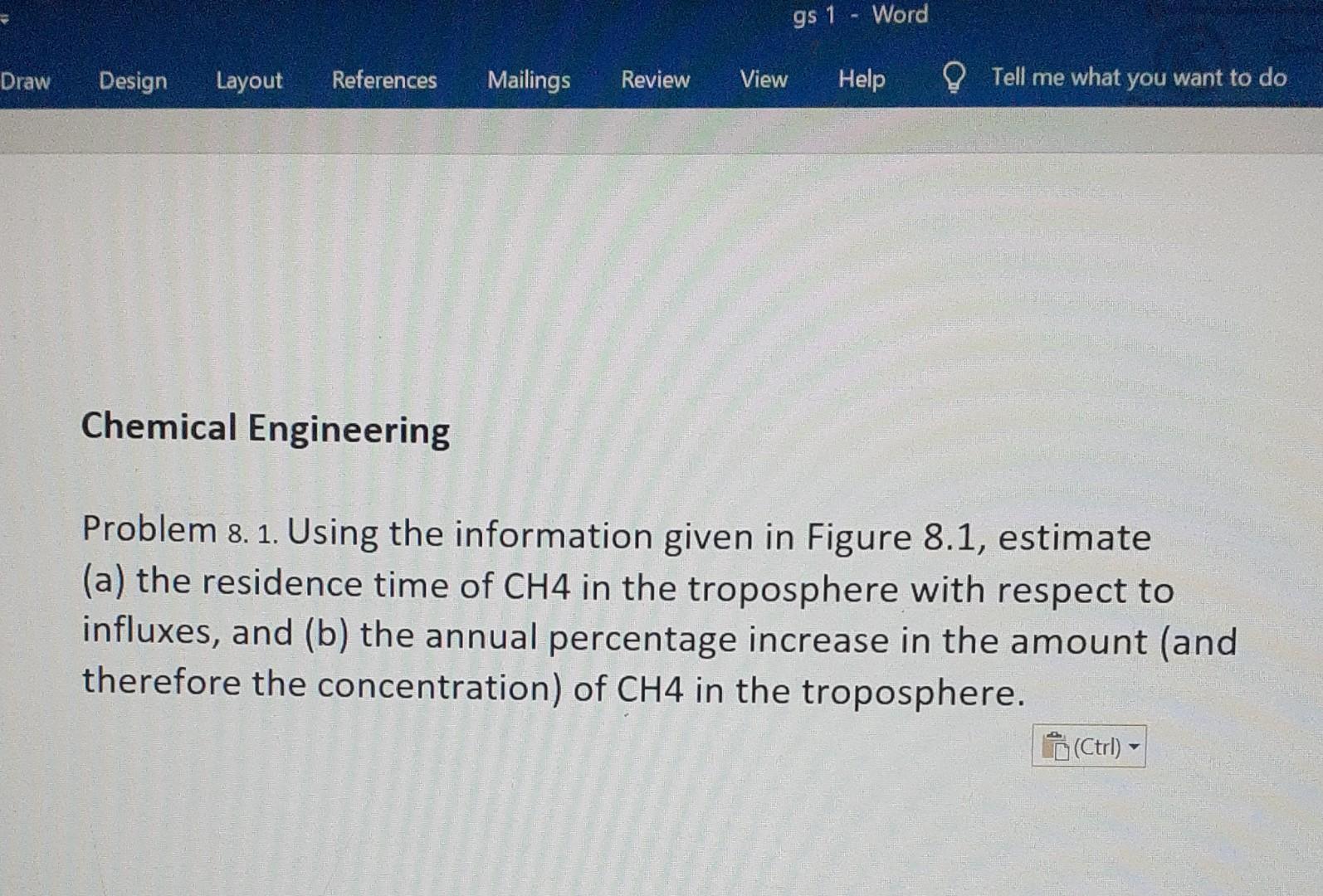
To the stratosphere To tho stratosphere 50 HCHO 17 0 OH andation 240 00 1200 g protokon OH Caddebon CO2 1750.000 TCW 20 CHA 2500 T310) Ww doption OCEANS 10040/20 525 80 22 340 300 100 105 110.000.000 5000 20.001) x pas 7 Domestic Rice Landhits animals felds Industrias Oodation Transportation Respiration Fossil fuel Combustion etc ol NMHC combustion and detrgus Comouston Termites Natural Bomass Coal Sois and decomposition Respiration Wetlands buming industries mnes CH4 Natural gas Vegetation Biomass Uptake Assimkaton Biomass Dissoluton into hydrate and ol buning by (photosynthesis) burning the oceans destabt ding soks and detarestation OCEANS AND lization leaka e CONTINENTS OCEANS FRESHWATER (1,000,000 Tg(c)] Figure 8.1. The principal sources and sinks of carbon-containing gases in the atmosphere. The numbers alongside the arrows are estimates of average annual fluxes in Tg(C) per year, various degrees of uncertainty, some quite large, are associated with all of the Auxes. The numbers in square brackets are the total amounts of the species in the atmosphere. gs 1 Word Draw Design Layout References Mailings Review View Help Tell me what you want to do Chemical Engineering Problem 8. 1. Using the information given in Figure 8.1, estimate (a) the residence time of CH4 in the troposphere with respect to influxes, and (b) the annual percentage increase in the amount (and therefore the concentration) of CH4 in the troposphere. (Ctrl)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


