Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve A liquid stream consisting of 12.0 mole% n-butane and the balance a heavy nonvolatile hydrocarbon is fed to the top of a stripping
please solve 
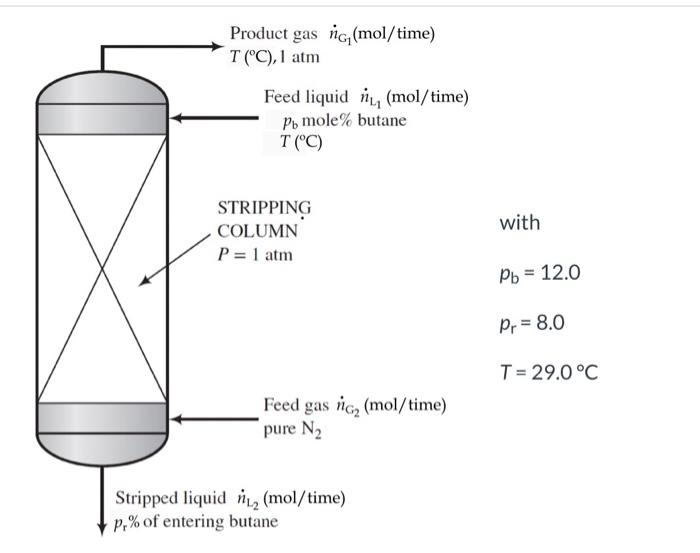

A liquid stream consisting of 12.0 mole% n-butane and the balance a heavy nonvolatile hydrocarbon is fed to the top of a stripping column, where it is contacted with an upward-flowing stream of nitrogen. The residual liquid leaves the bottom of the column containing all of the heavy hydrocarbon, 8.0% of the butane entering the column, and a negligible amount of dissolved nitrogen. Product gas ng (mol/time) T (C), 1 atm Feed liquid (mol/time) po mole% butane T(C) with STRIPPING COLUMN P= 1 atm Pb = 12.0 Pr = 8.0 T= 29.0C Feed gas ng, (mol/time) pure N2 Stripped liquid 1.2 (mol/time) p,% of entering butane The highest possible butane mole fraction in the exiting gas is that in equilibrium with the butane in the entering liquid (a condition that would require an infinitely tall column to achieve). Using Raoult's law to relate the mole fractions of butane in the entering liquid and exiting gas, calculate the molar feed-stream ratio (mol gas fed/mol liquid fed) corresponding to this limiting condition. n nt = 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


