Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve A,B,C for a like. must show all work Forming Moon For In-class problem #1, we calculated the minimum temperature of air that could
please solve A,B,C for a like. must show all work 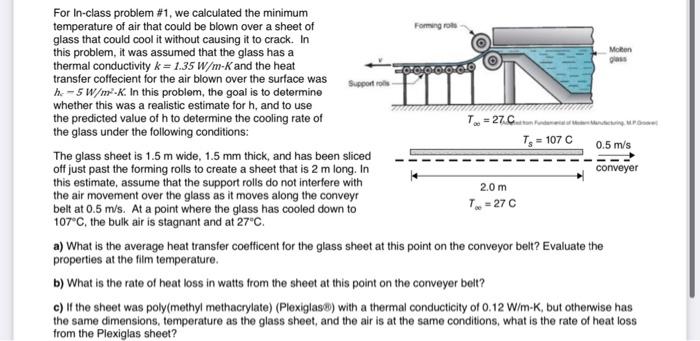
Forming Moon For In-class problem #1, we calculated the minimum temperature of air that could be blown over a sheet of glass that could cool it without causing it to crack. In this problem, it was assumed that the glass has a thermal conductivity k = 1.35 W/m-K and the heat transfer coffecient for the air blown over the surface was Supportrols h: -5 W/m-K. In this problem, the goal is to determine whether this was a realistic estimate for h, and to use the predicted value of h to determine the cooling rate of T = 27.c. the glass under the following conditions: T = 107C 0.5 m/s The glass sheet is 1.5m wide, 1.5 mm thick, and has been sliced off just past the forming rolls to create a sheet that is 2 m long. In conveyer this estimate, assume that the support rolls do not interfere with 2.0 m the air movement over the glass as it moves along the conveyr belt at 0.5 m/s. At a point where the glass has cooled down to T = 27C 107C, the bulk air is stagnant and at 27C. a) What is the average heat transfer coefficent for the glass sheet at this point on the conveyor belt? Evaluate the properties at the film temperature. b) What is the rate of heat loss in watts from the sheet at this point on the conveyer belt? c) If the sheet was poly(methyl methacrylate) (Plexiglas) with a thermal conducticity of 0.12 W/m-K, but otherwise has the same dimensions, temperature as the glass sheet, and the air is at the same conditions, what is the rate of heat loss from the Plexiglas sheet 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


