Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE SOLVE ALL PARTS 1. The reaction below is carried out under several sets of conditions enumerated below. Calculate the fraction of nitrogen reacted in
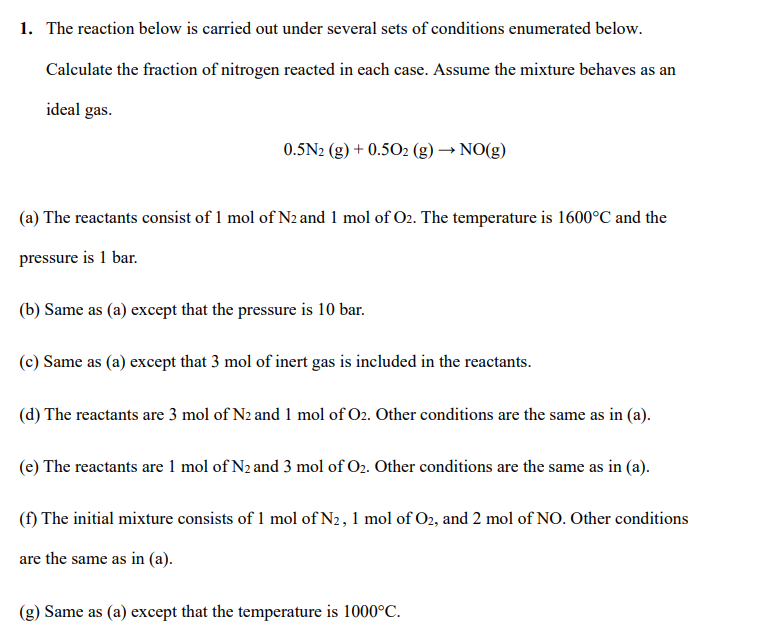
PLEASE SOLVE ALL PARTS
1. The reaction below is carried out under several sets of conditions enumerated below. Calculate the fraction of nitrogen reacted in each case. Assume the mixture behaves as an ideal gas. 0.5N2(g)+0.5O2(g)NO(g) (a) The reactants consist of 1mol of N2 and 1mol of O2. The temperature is 1600C and the pressure is 1 bar. (b) Same as (a) except that the pressure is 10 bar. (c) Same as (a) except that 3mol of inert gas is included in the reactants. (d) The reactants are 3mol of N2 and 1mol of O2. Other conditions are the same as in (a). (e) The reactants are 1mol of N2 and 3mol of O2. Other conditions are the same as in (a). (f) The initial mixture consists of 1molofN2,1mol of O2, and 2mol of NO. Other conditions are the same as in (a). (g) Same as (a) except that the temperature is 1000CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


