Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE SOLVE ALL PARTS 3. Synthesis gas can be produced by the catalytic re-forming of methane with steam. The reactions are: CH4(g)+H2O(g)CO(g)+3H2(g)CO(g)+H2O(g)CO2(g)+H2(g) Assume equilibrium is
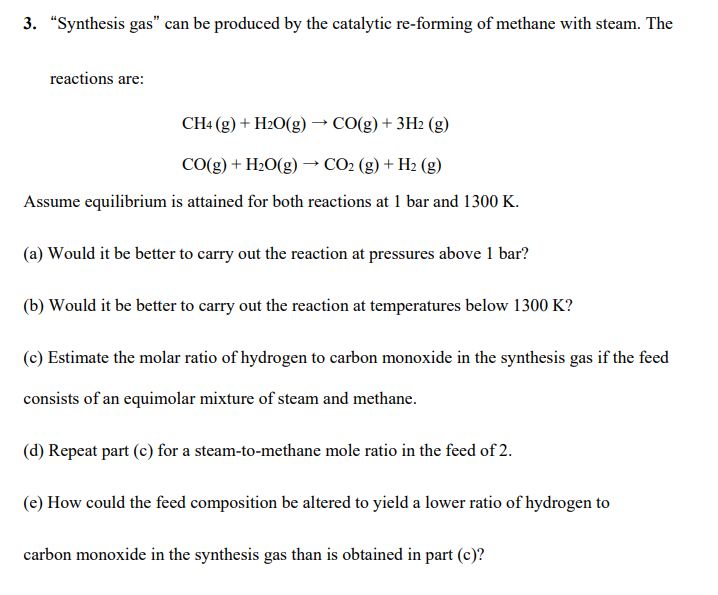
PLEASE SOLVE ALL PARTS
3. "Synthesis gas" can be produced by the catalytic re-forming of methane with steam. The reactions are: CH4(g)+H2O(g)CO(g)+3H2(g)CO(g)+H2O(g)CO2(g)+H2(g) Assume equilibrium is attained for both reactions at 1 bar and 1300K. (a) Would it be better to carry out the reaction at pressures above 1 bar? (b) Would it be better to carry out the reaction at temperatures below 1300K ? (c) Estimate the molar ratio of hydrogen to carbon monoxide in the synthesis gas if the feed consists of an equimolar mixture of steam and methane. (d) Repeat part (c) for a steam-to-methane mole ratio in the feed of 2. (e) How could the feed composition be altered to yield a lower ratio of hydrogen to carbon monoxide in the synthesis gas than is obtained in part (c)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


