Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve all parts of the question using the equations and table provided. B1. A stream of pyrolysis gas needs to be separated by distillation.
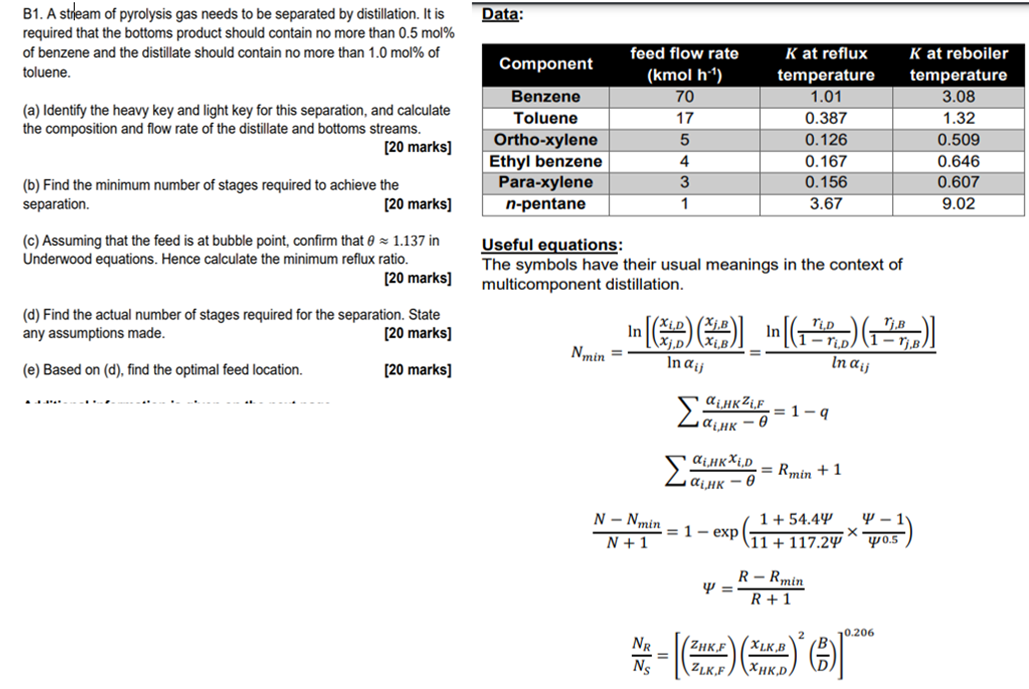
Please solve all parts of the question using the equations and table provided.
B1. A stream of pyrolysis gas needs to be separated by distillation. It is required that the bottoms product should contain no more than 0.5 mol % of benzene and the distillate should contain no more than 1.0 mol % of toluene. (a) Identify the heavy key and light key for this separation, and calculate the composition and flow rate of the distillate and bottoms streams. [20 marks] (b) Find the minimum number of stages required to achieve the separation. [20 marks] (c) Assuming that the feed is at bubble point, confirm that 0 1.137 in Underwood equations. Hence calculate the minimum reflux ratio. [20 marks] (d) Find the actual number of stages required for the separation. State any assumptions made. [20 marks] (e) Based on (d), find the optimal feed location. [20 marks] Data: feed flow rate Component K at reflux temperature (kmol h.) 70 1.01 Benzene Toluene 17 0.387 5 0.126 4 0.167 Ortho-xylene Ethyl benzene Para-xylene n-pentane 3 0.156 1 3.67 Useful equations: The symbols have their usual meanings in the context of multicomponent distillation. Xi,D TLD Tj,B In [(x)(x)] [() (27)] In Nmin = In ajj In ij = 1-q HKZLF HK-0 iHKXi,D =Rmin +1 N-Nmin = 1-exp 1 + 54.44 11 + 117.24 N + 1 Y = R-Rmin R+1 10.206 *-**** NR (ZHK,F XLK,B = ZLK.F XHK,DA X 4-1 40.5 K at reboiler temperature 3.08 1.32 0.509 0.646 0.607 9.02Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


