Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve all this question 2- The equation for the reaction in rocket engines is: C2H8N2(l)+N2O4(g)N2(g)+CO2(g)+H2(g) 1) 45.0g of C2H8N2 and 75.0g of N2O4 are
please solve all this question 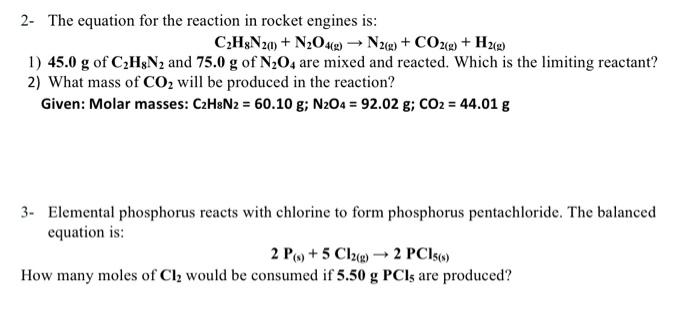
2- The equation for the reaction in rocket engines is: C2H8N2(l)+N2O4(g)N2(g)+CO2(g)+H2(g) 1) 45.0g of C2H8N2 and 75.0g of N2O4 are mixed and reacted. Which is the limiting reactant? 2) What mass of CO2 will be produced in the reaction? Given: Molar masses: C2H8N2=60.10g;N2O4=92.02g;CO2=44.01g 3- Elemental phosphorus reacts with chlorine to form phosphorus pentachloride. The balanced equation is: 2P(s)+5Cl2(g)2PCl5(s) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


